![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
90 Cards in this Set
- Front
- Back
|
Basal Ganglia Components
|
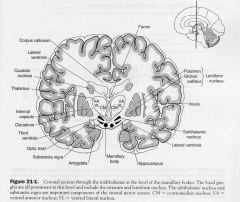
- Caudate Nucleus
- Putamen - Globus Pallidus - Subthalamus - substantia nigra |
|
|
Striatum
|
- caudate nucleus & putamen
|
|
|
Lentiform Nucleus
|
GP & Putamen
|
|
|
Corpus Striatum
|
lentiform nucleus and caudate nucleus
|
|
|
Claustraum
|
between lentiform and caudate nucleus
- recipricaol connections between the sensory cortices |
|
|
Striatal (EP) Motor System
|
F: initiation & execution of somatic motor activity
+ automatic stereotyped postural & reflex motor activity (e.g arm swing) |
|
|
Strucutres of Striatal Motor System
|
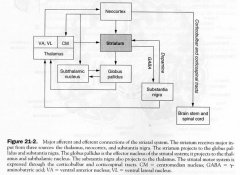
1. neocortex
2. striatum 3. GP 4. Sunthalamic Nucleus 5. Substantia nigra 6. Thalamus |
|
|
Parkinson's Disease
|
degenerative disease affecting substantia nigra & its projections to SN.
1. Results, in depletion of DA in SN & striatum as well as loss of melanin-containg dopaminergic neurons in the SN 2. SIGNS: bradykinesia, stooped posture, shuffling gait, cogwheel rigidity, pill-rolling tremor, masked facies - lewy bodies found in melanin-containing neurons of the SN. Progressive supranuclear palsy assoc. with PK. 3. Tx: L-Dopa -surgical; pallidotomy (rigidity) & ventral thalomotomy (tremor) |
|
|
Major NTs of Striatum
|
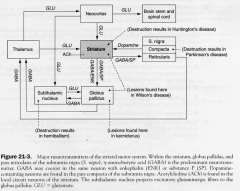
|
|
|
Methylphenyltetrahydropyridine (MPTP)- induced PK
|
MPTP analogue of meperidine and it destroys DA neurons in SN
|
|
|
Huntington's Disease
|
Chorea
- inherited autosomal dominant movement disorder - single gene defect of Chrom. 4 1. Degeneration of Cholinergic & GABAergic neurons of striatum + gyral atrophy in the frontal & temporal lobes 2. Glutamate excitotoxicity. in HD Glutamate binds NMDA receptors influx of Ca causing cell death 3. Signs; choreiform movements, hypotonia, progressive dementia |
|
|
Syndenham's Chorea (St. Vitus Dance)
|
most common chorea overall
- coomon in girls after a bout of rheumatic fever |
|
|
Chorea Gravidarum
|
2nd trimester pregnancy and many with a Hx of Syndenham's Chorea
|
|
|
Hemiballism
|
- movement disorder due to a vascular lesion in subthalamus
SIGNS: contralateral violent flining (ballistic) movements of one or both extermities |
|
|
Hepatolenticular Degeneration
|
(Wilson's Disease)
- autosomal recessive disorder caused by a defect in the metabolsim of copper - gene locus on Chrom. 13 1. SIGNS: choreiform, rigidoty, wing-beating tremor 2. LESIONS: lentiform nucleus, Cu depostion in limbus of cornea (corneal Kayser-Fleischer Ring) + deposition of Cu in liver leads to multi-lobe cirrohosis 3. Psychiatric Sx 4. Dx; low serum ceruloplasmin, elevated urinary excretion of Cu & Inc. Cu conc. in a liver biopsy 5. Tx; penicillamine, a chelator |
|
|
Tardive Dyskinesia
|
syndrome of repetitive choreic movement that affect the face & trunk
- usually due to phenothiazines, butyrophenones, metoclopramide |
|
|
Motor Pathways
|
MAKERS: M1, brainstem, spinal cord
DESIGNERS: M2, basal ganglia (+cerebellum) Clinically defect: MAKER = no voluntary moves DESIGNER: abnormal voluntary move |
|
|
CAudate and Putamen
- cell types - pathways |

1 functional unit (striatum)
1. SPINY cells: projection cells GABA_SP GABA_Enk 2. Aspiny Cells: interneurons GABA ACh IN: cortex and SNc OUT: GP |
|
|
Globus Pallidus
|
STRUCTURE
• 2 segments: GPi, GPe • GABA PATHWAYS • in: CPu, Sub out: thalamus, PpT, Sub STRUCTURE & PATHWAYS Subthalamus |
|
|
Substantia Nigra (SN)
|
STRUCTURE
• SNr: pars reticulata (~GPi), GABA- • SNc: pars compacta (black), dopamine+/- PATHWAYS - SNr in: CPu, Sub out: thalamus, PpT, Sub - SNc in: cortex out: CPu |
|
|
Subthalamus (Sub)
- frisky |
STRUCTURE
• below thalamus • Glu+ PATHWAYS • in & out: GP |
|
|
Pedunculopontine (PpT
|
STRUCTURE
• Ach+ / Glu+ PATHWAYS in: GP out: SpC |
|
|
Circulatory
|
1. Cortex focus movement
2. order Basal ganglia help a) activate some thalamic/PpT Circuits (DIRECT) B) inhibit some (INDIRECT) |
|
|
Direct Pathway
|
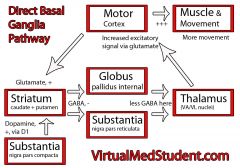
|
|
|
Indirect Pathway
|
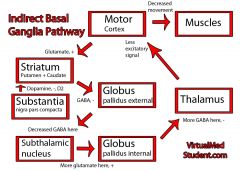
|
|
|
Substantia Nigra Compacta Pathways
|

OVERALL FUNCTION
• helps cortex activate: - D, InD; in active zone - SPOT LIGHT brighter • dopamine: same 2 paths - D1r: excite - D2r: inhibit - hallmark of PKD; too much inhibition of the thalamus (by subthalamic cortex) - DA is the only thing that switches the InD pathway off |
|
|
Consequence of no SNc PAthway
|
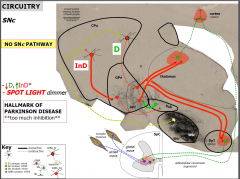
|
|
|
Functions of the basal ganglia
|
• 3 speculations; all linked
(i) Focus: open some gates, close others (ii) Plan: linking areas, planning; idea to expression (iii) Programmes: start/stop and store |
|
|
PK causes
|
• genetics (15%); head trauma; virus (influenza); toxins (MPTP)
• SNc cell death; progressive (continued toxin/gene or glial attack?) PROTECTORS: anti-oxidants, coffee, smoke |
|
|
PK signs
|

• tremor* (rest)
• rigidity* (hypertonia), akinesia* (no), bradykinesia* (slow) - face, gait, posture (reflex), speech, write • other: ANS, smell, arousal/moods |
|
|
PK pathology
|

• âSNc (dopamine): 50-70%
• Lewy body: αsynuclein • mitochondria dysfunction (âATP, áreactive oxygen species) • áInD*, thus áGP/Sub: HALLMARK **importance of Sub** |
|
|
Mechanisms
|
TREMOR
• +GP TO - thalamus (abnormal oscillations; DECGP vs DECcerebellum inputs) RIGIDITY/AKINESIA/BRADYKINESIA (RAB) • +GP à -PpT (âα cell; AKINESIA/BRADYKINESIA) (âinterneurone à áα cell; RIGIDITY) |
|
|
PK Treatment
|
• DRUGS: 1st line - GOLD standard
- Ldopa - Da agonist (eg, Bromocriptine) - MaOI (eg, Selegiline, COM-T inhibitors) • SURGERY: 2nd line - disrupt abnormal circuit (combination with drugs) - lesion/deep brain stimulation (DBS; ~100Hz) - targets: thalamus, GP, Sub* |
|
|
Future Treatments
|
• STIMULATE MITOCHONDRIA: anti-oxidants?
• TRANSPLANTS: foetal SNc • STEMS: new cells • GENE THERAPY: δ phenotype |
|
|
Parkinsonism
|
•
Alpha-synucleinopathies – Parkinson disease – Dementia with Lewy bodies – Multiple system atrophy • Non-a-synucleinopathies – Tauopathies • Progressive supranuclear palsy • Corticobasal degeneration • Frontotemporal dementia with parkinsonism-17 • Guam-Parkinson-dementia complex • Dementia pugilistica – TDP-43 proteinopathies • FTLD-TDP – Non-specific degeneration in substantia nigra • Other – Infarcts – Drug-induced (dopamine antagonists, calcium channel blockers, sodium valproate, kava) |
|
|
Clinical features of PD
|
Clinical features of PD
• Major features – Rigidity – Bradykinesia – Postural instability – Resting tremor (5 Hz) • Good differentiator, but absent in up to 25% of PD • Additional features – Dysphagia – Autonomic dysfunction – Dementia (7 times more common) – Depression in one-quarter – Hyposmia – Visual hallucinations (late) – Micrographia |
|
|
DX PK
|
•
Combination to best differentiate PD – Resting tremor – Asymmetry of symptoms and signs – Good response to levodopa • No biological marker to unequivocally diagnose the disease – But increased CSF alpha-synuclein can help diagnose PD • Autopsy diagnosis remains the gold standard – Only way to make a “definite” diagnosis of PD – 10-25% of diagnoses of PD found to be incorrect in autopsy studies • Under-diagnosis by 25% in door-to-door studies • In most cases, the diagnosis of PD can be made on clinical grounds – Brain imaging to exclude other conditions – Dopa-PET or SPECT used occasionally to show dopa uptake in striatum |
|
|
Epidemiology of PD
|
•
Age of onset usually 60-70 years • Prevalence increases exponentially with age between 65 and 90 years – 3% of people over 65 years have PD – Over the age of 80, up to 10% of people have PD – Early onset (less than 50 years) in only 4% • Mortality 4x higher than controls |
|
|
Loss of neurons in PD
|
•
Substantia nigra in midbrain – Dopaminergic neurons (leads to loss of dopamine from the striatum) – Need to lose about 60% of cells before symptoms appear – Most severe loss ventro-lateral • Locus ceruleus in pons: noradrenergic neurons • Raphe neurons: serotonergic neurons • Basal nucleus and mesencephalic locomotor area: cholinergic neurons • Autonomic neurons (spinal intermediolateral sympathetic and gut parasympathetic) |
|
|
Lewy bodies and neurites in PD
|
•
Lewy bodies – Intraneuronal: round, pink, hyaline core and pale-staining peripheral halo – One neuron may contain multiple Lewy bodies – Found in surviving neurons in the • Substantia nigra • Locus ceruleus • Dorsal motor nucleus of vagus – Found in about 5% of elderly brains – Composed of neurofilaments, densely packed (in core) and radially arranged (in halo) peripherally – Specific protein: alpha-synuclein.Other proteins trapped passively – May be a manifestation of cell protective mechanisms • Lewy neurites (in neuronal processes) |
|
|
What is the cause of PD?
|
•
Favoured hypothesis: an endogenous or exogenous toxin + a genetic susceptibility • In younger patients a single gene defect may be responsible • Some think gene changes will be found even in “sporadic” PD |
|
|
Environmental factors in PD
|
•
First described during the Industrial Revolution 180 years ago, therefore ? a new exogenous toxin (but historical clinical descriptions of PD before this time makes an industrial exposure less likely) • Possible risk factors – Weak associations with rural living, head injury, drinking well water, exposure to pesticides and herbicides • Possible protective effects – Tobacco smoke – Caffeine |
|
|
Pesticides in PD?
|
•
Epidemiological evidence • Polymorphisms in glutathione transferase (a pesticide-detoxifying enzyme) have been associated with PD • Rotenone: a mitochondrial complex 1 inhibitor – Little effect given via injection – If given intra-gastrically, get a-synuclein accumulation in enteric, dorsal vagal, and substantia nigra neurons (with loss of SN neurons) – Consistent with hypothesis that a toxic agent can enter the CNS via the enteric nervous system |
|
|
MPTP in PD
|
•
Methyl-phenyl-tetrahydro-pyridine (MPTP) produces selective nigral neuronal loss • People taking heroin or meperidine containing MPTP develop a PD-like disease • Astrocytic monoamine oxidase B turns MPTP into a more reactive product, MPP, that is absorbed by dopamine transporters and inhibits mitochondrial complex 1 • Slowly progressive loss of neurons can occur after a single dose of MPTP • The herbicide paraquat, similar in structure to MPP, damages mouse dopaminergic neurons via free radicals |
|
|
Post-encephalitic parkinsonism
|
•
Encephalitis lethargica (1915-1927) • Nature of infectious agent remains unknown • Parkinsonism after a latent period of 10 years • L-DOPA responsive • Widespread neurofibrillary tangles • No Lewy bodies |
|
|
Genetics in PK
|
•
Late-onset PD was initially thought to be purely sporadic • But about 20% of people with PD have a first-degree relative with the disease. This was thought to be due to a common environmental exposure • Twin studies have been difficult because PD genes have incomplete penetrance and show wide variations in phenotypes in a single family • A number gene mutations have now been found in L-dopa responsive parkinsonism • Large association studies looking for genetic susceptibility have implicated a number of common variations in single nucleotide polymorphisms • Most genetic components of PD remain to be discovered |
|
|
Genetic factors in PD
|
•
Alpha-synuclein – Point mutations and triplications in familial PD – Gain of toxic function mutations – Variations are associated with PD susceptibility • LRRK-2 – 4% hereditary, 1% sporadic PD – Gain of function mutations – Variations are associated with PD susceptibility • Loss of function mutations in parkin (second most common), DJ-1, PINK1 and ATP13A2 cause early onset recessive parkinsonism • Parkin and PINK1 share same mitochondrial pathway • Parkin and alpha-synuclein affect ubiquitin proteasome system |
|
|
Vesicles in PD
|
•
Mutations in the alpha-synuclein gene • Altered alpha-synuclein function leads to impaired vesicular binding • This leads to impaired dopamine release from vesicles and increased dopamine in the cytoplasm (which is toxic) |
|
|
Misfolded proteins
|
•
Alpha-synuclein aggregates in PD (Lewy body) • Park2 (Parkin) and DJ1 are involved in the ubiquitin-proteasome pathway • Over-expression of alpha-synuclein inhibits the proteasome • Rats given proteasomal inhibitors get features of PD |
|
|
Mitochondria in PD
|
•
40% decrease in mitochondrial complex 1 in the substantia nigra in PD (and platelets) • MPTP affects complex 1 • Mitochondrial function is affected by decrease in PINK1, DJ1 and Parkin activity • Complex 1 inhibition causes alpha-synuclein aggregates similar to Lewy bodies • When complex 1 is faulty, energy production falls and free radicals rise |
|
|
Free radicals in PD
|
•
Free radicals lack an electron and grab electrons from (oxidise) other molecules – Injure membranes (peroxidation) – Injure genetic material – Form new free radicals • Excess free radicals are suspected in PD because – Decreased mitochondrial energy – Increased lipid peroxidation in the substantia nigra in PD – High levels of iron in the substantia nigra – Dopamine can promote free radical synthesis |
|
|
Excitotoxins in PD
|
•
The glutamate-producing subthalamic nucleus is overactive in PD • Glutamate operates on the NMDA receptor, which increases intracellular calcium • Dopaminergic neurons that contain the calcium-binding protein, calbindin, are preserved in PD |
|
|
Recent advancements in PK
|
•
It may be possible to detect synuclein aggregates in gastrointestinal tissue through biopsy samples taken during gastroscopy or colonoscopy • Abnormal striatal α-synuclein can trigger many of the features of sporadic PD • 7 Tesla MRI provides improved visualisation of the substantia nigra and a more precise characterisation of its shape and structure • Strengthening exercises and the training of sensorimotor agility improve PD severity to the same extent as that achieved with levodopa treatment |
|
|
Risk Factors for PK
|
- family Hx of PK or tremor
- preceding constipation - prior mood disorder - exposure to pesticides - previous head injury - rural living - beta-blocker use - farming occupation - well water drinker |
|
|
Protective factors for PK
|
- smoking (by 36%)
- coffe drinking - prior HT - NSAID use - CCB - EtOH |
|
|
Stages of Pk
|

|
|
|
Parkinsonism
|
Parkinsonism; anything that looks like it
Diseases with similar features – Progressive supranuclear palsy – Diffuse lewy body disease Drug effects – Dopamine blocking agents Acute parkinsonian features Tardive syndromes |
|
|
Types of tremor
|
Rhythmic movement;
-Damped rhythmic systems Physiological tremor Enhanced physiological tremor Essential tremor (benign familial tremor) Intention tremor (cerebellar disease) And……. RESTING TREMOR |
|
|
Resting tremor
|
Not really…. “postural tremor” is a better term
The tremor of Parkinsons Disease –Unilateral, usually hand –Appears when the limb is not subject to conscious control –Related to sub-conscious motor activity (“posture”) –Generally slower than physiological and essential tremors –Not the big issue! |
|
|
Hypokinesis
|
The palsy of “the shaking palsy” Paucity of movement rather than slowness Loss of multiple small motor tasks; grooming, postural adjustments Trouble with rapidly sequenced repetitive activities: cleaning teeth, writing Closely related to symptoms |
|
|
Rigidity
|
Normal strategy: minimal energy use Co-contraction: voluntary in normal life – Involuntary in Parkinsons The origin of limited arm swing Consumes energy Causes pain |
|
|
Clinical Features: Late
|
Loss of righting reflexes Non-motor manifestations Sleep (‘acting out’ may be early) Autonomic constipation, hypotension Neuropsychiatric depression, dementia Pain |
|
|
Loss of righting reflexes
|
Complex multi-neuronal pathways Term derives from pathways studied in cats Anything that stops you falling over Borderland between conscious and unconscious behaviour Leads to loss of independence |
|
|
Autonomic trouble
|
Autonomic failure is rare Autonomic symptoms are common – Constipation – Orthostatic hypotension – Frequent micturition – Sudomotor dysfunction – Greasy skin |
|
|
Depression
|
Common – 20% major – 20% minor May be related to Dopamine’s role in reward systems Is more likely in people who are severely affected |
|
|
Dementia
|
Risk is 2-6 times an age matched population Prevalence is 20-40% Risk factors – Age – Male sex – Severity of Parkinsons Disease – Depression – Low educational level |
|
|
SN in PD
|

|
|
|
Alpha-Synuclein
|
140 amino-acid protein Function unknown Found in many nerve cells Dysfunctional folding lead to aggregation in beta-pleated sheets Aggregations seen in neurites and as Lewy bodies Mutations in the alpha-synuclein gene lead to familial Parkinsons Disease |
|
|
Lewy Body
|
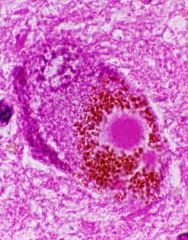
Intra-neuronal Acidophilic Dense core Pale halo Pathological hallmark of Parkinsons Disease Seen in other diseases (and aged normal brains) Large numbers in Lewy Body disease |
|
|
Staging: Clinical
|
Staging: Clinical
Hoen and Yahr i Unilateral, mild non-disabling ii Bilateral, minimal disability, some gait trouble iii Significant slowing, loss of righting reflexes, moderate disability iv Severe, still walking, cannot live alone v Cachectic, invalid, constant nursing care |
|
|
Staging: Pathological
|
Staging: Pathological
Heiko Braak 1-2, (pre-symptomatic) medulla, pontine tegmentum, olfactory bulb 3-4, (symptomatic) substantia nigra, limbic system 5-6, (late) neocortex |
|
|
Catecholamine Synthesis
|
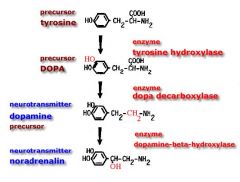
Catecholamine (doubly hydroxylated benzene ring with an ethylamine substitution) Starting point is Tyrosine Includes dopamine, adrenaline and nor-adrenaline |
|
|
CNS Dopamine Actions
|
Acts at the post-synaptic dopamine receptor Is broken down by monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT) Is taken back up into the pre-synaptic terminal and re-packaged |
|
|
Dopamine Metabolism
|
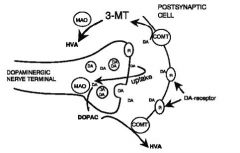
|
|
|
Dopa Decarboxylase
|
Widely distributed enzyme
Converts 99% of an oral dose of l-dopa to dopamine High levels of peripheral dopamine are very nauseating (act at the chemo-receptor trigger zone) Dopa decarboxylase can be inhibited by drugs which are not able to cross the blood-brain barrier |
|
|
Peripheral DDIs
|
–
Carbidopa (Sinemet) – Benserazide (Madopar) – Domperidone (separate agent) |
|
|
Dopamine Receptors
|
Two classes: D1 and D2
D1: Linked to adenylate cyclase. All post-synaptic. Alter membrane permeability D2: Not linked. Pre and post-synaptic. The main site of clinical pharmacology |
|
|
D1 family
|
D1,5
|
|
|
D2 Family
|
D2,3,4
|
|
|
Dopamine Agonists
|
Dopamine (D1 and D2) Bromocriptine (D2) Apomorphine (D2) Pramipexole (D2) Pergolide (D2) Cabergoline (D2) |
|
|
Dopamine Antagonists
|
Clozapine (D1 & D2) Anti-Psychotics Chlorpromazine (D2) Haloperidol (D2) Risperidone (D2) Domperidone (D2) Anti-nauseants Metoclopramide (D2) Prochlorperazine (D2) |
|
|
Other Agents Acting at the Dopaminergic Synapse
|
Amphetamines (facilitate dopamine release) Cocaine (inhibits dopamine re-uptake) MAO inhibiting antidepressants (prevent dopamine catabolism) Tricyclic anti-depressants (inhibit dopamine re-uptake) |
|
|
Pharmacotherapy of P.D.
|
A combination of L-dopa and a dopa decarboxylase inhibitor is the mainstay of treatment (Madopar, Sinemet) Response to this combination is required for diagnosis Nausea is the commonest problem. Use a low dose, with food, titrate up gradually. |
|
|
Problems with Levo-Dopa I
|
Hypotension (esp. orthostatic) –Less with time and slow titration –Severe suggests an alternative diagnosis Dyskinesia –Appears after years –Usually dynamic but may be fixed –Common in “Parkin” gene carriers Psychiatric disturbance –Disturbed dreams –Paranoid psychosis –Formed visual hallucinations (these habituate) –Gambling, hypersexuality, punding Loss of efficacy (is levo-dopa the problem?) –Shortened duration –On/Off phenomenon |
|
|
Direct Dopamine Agonists
|
These act on the post synaptic receptor (D2) Bromocriptine, Pergolide, Cabergoline Theoretical advantage (bypass sick or dead neurones) Much more expensive than L-dopa/DDI In fact behave much like L-dopa (nausea, hypotension, psychiatric disturbance) |
|
|
Indications for Dopamine Agonists
|
Dyskinesia (usually in combination with L-dopa/DDI to enable reduced dosage) Longer t1/2 may smooth fluctuations |
|
|
Amantadine
|
Older agent (use declining): inhibits dopamine re-uptake and facilitates release Also acts at Phencyclidine site on NMDA receptor Benefit is often transient (6-12 months) Renal excretion (cannot use in renal failure) Hard to get people off it |
|
|
Anti-Cholinergic Drugs
|
Striatum seems in some way to compare dopaminergic and cholinergic inputs Reducing cholinergic input is mildly beneficial in P.D. Noted when belladonna was used to treat hypersalivation in P.D in the 19th Century Major benefit is on tremor Side effects –Paralyse ciliary muscle –Urinary retention –Constipation –Impaired sweating –Delirium and hallucinations Withdrawal must be slow |
|
|
The MPTP Story
|
A chemist in California attempting to synthesize Demerol for recreational use Inadvertently produced MPTP (Methyl Phenyl Tetra Hydro Pyridine) Drug addicts using the product developed profound irreversible Parkinsonism within hours to days. |
|
|
Deprenyl
|
Inhibits MAO-B Slows metabolism of Dopamine and produces a mild therapeutic effect MAO-B is required for activation of MPTP to MPP+ which produces Parkinsonism Thought it might prevent progression: didn’t – (suicidal inhibitor, study measured the therepeutic benefit) |

