![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
178 Cards in this Set
- Front
- Back
|
How many alpha helices do alpha chains and beta chains of hemoglobin have? |
alpha: 7 alpha helices Beta and Mb: 8 alpha helices |
|
|
How many residue interactions do alpha1-beta1 have? Alpha1-beta2? |
35 for alpha1-beta1 19 for alpha1-beta2 |
|
|
Both Mb and Hb are saturated at lung partial pressures. What is their oxygen saturation at tissues? |
Myoglobin is still saturated at tissue partial pressures and Hb is not fully saturated |
|
|
The iron in deoxy Hb has how many ligand interactions |
5 |
|
|
The iron in oxy-Hb has how many ligand interactions |
6 |
|
|
Which state has the higher oxygen affinity |
relaxed (oxy-Hb) state |
|
|
How does pH and CO2 concentration affect oxygen affinity of hemoglobin |
both shift the curve to the right so that in more acidic conditions Hb releases more oxygen. |
|
|
What is the effect of 2,3 BPG |
it binds to deoxy HB and stabilizes the tense form of Hb making it harder of oxygen to bind. This is an adaptation to low oxygen environments and leas to oxygen unloading. |
|
|
What is one of the roles of the distal histidine relating to restriction of the sixth coordination site. What affect does this have on carbon monoxide binding? |
The distal histamine restricts the 6th coordination site so that ligands must bind at an angle. This decreases the affinity of anything that binds. Without this Carbon monoxide would bind with 25,000x more affinity than oxygen rather than just 200x more affinity |
|
|
How does the distal histidine protect iron |
Ferrous iron (2+) is protected from oxidation to ferric (3+) |
|
|
iron 3+ forms what kind of hemoglobin. What do our cells have to fix this? |
methemoglobin. Methemoglobin reductase can reduce ferric back to ferrous iron |
|
|
If you are poisoned by an oxidant that forms methemoglobin what can you use to treat |
methylene blue also reduces ferric to ferrous iron. |
|
|
What genes are the alpha and beta clusters found on that code for globin chains used to make Hb? |
alpha is chromosome 16 beta is chromosome 11 |
|
|
Describe congenital Heinz body hemolytic anemia |
CHBA means that unstable hemoglobins are made that form heinz bodies in RBCs. |
|
|
High affinity variants of Hb cause what |
Familial erythrocytosis (polycythemia) because you produce more RBCs to account for the decreased oxygen delivery |
|
|
Low affinity variants of Hb cause what |
They can cause cyanosis but are generally asymptomatic due to normal oxygen delivery and therefore the body does not compensate. |
|
|
Describe M hemoglobins. What part of Hb is affected? Color changes? Oxygen affinity? |
proximal or distal histidines are affect. People look lavender blue and have chocolate brown blood but normal oxygen affinity |
|
|
Give three examples of amino acid derived hormones |
Catecholamines, serotonin, thyroxine |
|
|
Which hormone is a tripeptide |
TRH |
|
|
Small peptide hormones |
vasopressin and somatostatin |
|
|
Intermediate peptide hormones |
Insulin and PTH |
|
|
Protein and glycoprotein hormones |
Gonadotropins and TSH |
|
|
Cholesterol derived hormones |
Glucocorticoids, mineralcorticoids, androgens, estrogens and vitamin D |
|
|
Fatty acid derivative hormones |
prostaglandins and leukotrienes |
|
|
Phospholipid derivative hormone |
platelet activating factor |
|
|
Purine derived hormone |
Adenosine |
|
|
Gas hormone |
NO |
|
|
Describe how G-protein receptors work |
hormone binding causes displacement of GDP by GTP and dissociation of G-protein from the receptor and dissociation of alpha from the beta-gamma subunits |
|
|
Gs and Gi do what to adenylyl cyclase |
Gs stimulate adenylyl cyclase Gi inhibits adenylyl cyclase |
|
|
What does adenylyl cyclase do |
it converts ATP to cAMP |
|
|
cAMP stimulates _____ which ____'s proteins |
protein kinase A, phosphorylates |
|
|
How is cAMP turned off |
cAMP phosphodiesterase converts cAMP to 5'AMP |
|
|
How is calmodulin activated? What is its action |
It is activated by binding of four calcium. It influences many enzymes (activates or deactivates) |
|
|
How are DAG and IP3 formed |
A hormone binds an extra-cellular receptor which activates Gq the alpha subunit of which activates phospholipase C which cleaves PIP2 into DAG and IP3. IP3 causes ER to release calcium |
|
|
Describe JAK/STAT |
Hormone binding dimerizes JAK which phosphorylates STAT. STAT-P dimerize and enter the nucleus and activate transcription by binding to DNA. |
|
|
Describe cytosolic receptors. Give hormone examples |
Receptors in the cytosol are bound to heat shock proteins (HSPS). Hormone binding displaces the HSPS so the hormone-receptor complex can go to the nucleus and bind to the hormone response element HRE. The interaction turns on or turns off gene transcription
Sex Steroids |
|
|
Describe Nuclear receptors |
Receptors in the nucleus are not bound to HSPS. Hormone binding activates the receptor so that it can bind to HRE.
Thyroid, retinoids, VIT D |
|
|
What are the three glycoprotein hormones |
TSH, FSH and LH |
|
|
Describe the structure of glycoproteins |
They are all heterodimers with one alpha and one beta. Alpha subunit is the same across hormones so it is the beta subunit that differs and gives the hormone its activity. The beta subunit, however, is not active without the alpha subunit. |
|
|
Which organ is the master endocrine gland and what is its target organ |
the hypothalamus is the master and it targets the pituitary. |
|
|
Which hormones are released from the posterior pituitary? Where do the other hormones come from? |
Posterior: stores and secretes vasopressin and oxytocin
Anterior pituitary secretes all others ( TSH, PRL, ACTH, LH, FSH, GH) |
|
|
The thyroid gland makes and secretes ___ and ___ which is synthesized from ___ in a reaction catalyzed by ___. These hormones increase the BMR. |
thyroxine (T4) and thyronin (T3) thyroglobulin thyroperoxidase |
|
|
In target tissues ____ converts T4 to T3 |
Deiodinase |
|
|
Parathyroid hormone does what to serum calcium |
increases serum calcium |
|
|
Calcitonin is secreted by what and does what? |
secreted by thyroid C-cells and decreases serum calcium level |
|
|
Describe Atrial natriuretic peptide |
atrial muscle cells have ANP and is secreted when sodium chloride intake is increased and when the extracellular fluid volume expands. It induces sodium excretion, diuresis and inhibits aldosterone secretion. |
|
|
Pancreatic alpha cells, beta cells and delta cells each secrete what? What is the action of their secretions |
alpha: glucagon-increases blood glucose beta: insulin-decreases blood glucose delta: somatostatin-inhibits everything |
|
|
What does the adrenal medulla secrete. Explain how these hormones are synthesized |
Epi and NorEpi for fight or flight
Tyrosine -> dopamine -> NorEpi -> Epi |
|
|
Ghrelin |
appetite stimulation |
|
|
GLP-1 (glucagon-like peptide) |
sensitizes to insulin and inhibits glucagon |
|
|
CCK |
increases pancreatic digestive enzymes |
|
|
Pancreatic polypeptide |
Suppresses glucose-induced insulin secretion |
|
|
Gastrin |
Stimulate acid and pepsin secretion |
|
|
Secretin |
Stimulates release of bicarb and water |
|
|
VIP |
relaxes GI, inhibits acid and pepsin |
|
|
Neuropeptide tyrosine |
controls feeding behavior and energy homeostasis |
|
|
Leptin |
limits food intake and increases energy expenditure |
|
|
adiponectin |
increases insulin sensitivity and FA-oxidation |
|
|
Resistin |
induces insulin resistance |
|
|
What are the steroidogenic tissues? |
Adrenal cortex, testis and ovary |
|
|
What is the first step of steroidogenesis |
conversion of cholesterol to pregnenolone catalyzed by cholesterol side chain cleaving enzyme (SCC) (aka 17:20 lyase/desmolase) |
|
|
Where does conversion of testosterone to dihydrotestosterone take place? What enzyme does it? |
takes place in peripheral tissue through the action of 5 alpha reductase |
|
|
What hormones does the placenta secrete |
hCG, estrogen, progesterone |
|
|
The R in RAAS stands for ___ and is produced where? |
R-Renin and it is produced in the kidney |
|
|
Where is the active form of Vit D produced |
in the kidney |
|
|
What is Vit D3 involved in |
Calcium absorption |
|
|
What does the pineal glad produce? What is its action? |
melatonin is involved in circadian rhythm |
|
|
What is the effect of aging on the endocrine system |
aging slows down endocrine function |
|
|
Why would you maybe think twice about hormone replacement therapy |
it has serious side effects |
|
|
Zona glomerulosa synthesizes what hormone |
mieralcorticoids (aldosterone) which regulates sodium and potassium levels |
|
|
Which cells synthesize testosterone |
leydig cells |
|
|
What do sertoli cells synthesize to keep testosterone concentration high within testes |
Androgen-binding protein |
|
|
Briefly outline the RAAS |
Renin converts angiotensinogen to angiotensin I and ACE converts angiotensin I to angiotensin II |
|
|
What is the action of angiotensin II |
potent vasoconstrictor and stimulator of aldosterone production resulting in sodium retention, volume expansion and increase BP. |
|
|
What would a decrease in plasma calcium do to the PTH level |
decreased plasma calcium increases PTH release |
|
|
What does an increase in VIT D do to PTH levels |
Increased VIT D causes a decrease in PTH |
|
|
What two things can increase PTH release |
hypocalcemia or VIT D deficiency increase PTH |
|
|
Maximal PTH secretion is achieved when serum calcium levels reach what? |
serum calcium ≤1.15 mmol/L |
|
|
What are the actions of PTH. Which is fastest and which is larger |
kidney: reduces renal excretion of calcium (Fastest) (and increases phosphate excretion)
Bone: increases rate of bone dissolution (largest)
GI: indirectly through increasing synthesis of vitamin D which then promotes calcium absorption in the intestine
Net effect: serum calcium increased and serum phosphate decreased |
|
|
1,25 (OH)2-D3 (active form of vitamin D) is synthesized by what enzyme |
1 alpha hydroxylase |
|
|
Where does gluconeogenesis take place |
liver and kidney |
|
|
The irreversible steps of glycolysis must be circumvented by what enzymes? |
pyruvate carboxylase PEP carboxy kinase Fructose 1,6 bisphosphatase Glucose 6 phosphatase |
|
|
Which two enzymes are required to circumvent the action of pyruvate kinase |
pyruvate carboxylase and PEP carboxy kinse |
|
|
Pyruvate carboxylase creates oxaloacetate which needs to get across the mitochondrial membrane. How does this occur |
the malate aspartate shuttle where oxaloacetate is converted into malate and aspartate which can be transported across the membrane (aspartate with the help of cycling glutamate and alpha-ketoglutarate back and forth). Aspartate and malate can be rejoined into oxaloacetate in the cytoplasm |
|
|
What is the major control point of gluconeogenesis |
Fructos 1,6 bisphosphatase |
|
|
Which enzyme is only expressed in the liver |
glucose 6 phosphatase (which is why only the liver can regulate blood glucose, it is the only organ that can remove a phosphate so glucose can leave) |
|
|
How many high energy bonds are required to make one molecule of glucose |
6 high energy bonds per glucose |
|
|
Describe the cori cycle |
the cycle of lactate going to the liver and glucose going into erythrocytes and muscle. The RBCs have to give lactate to the liver b/c they don't have mitochondria so lactate is the end product of glycolysis for them |
|
|
Describe the alanine cycle |
alanine is exchanged for glucose |
|
|
alanine and lactate can be combined to form |
pyruvate |
|
|
Glucose can be formed from |
pyruvate (lactate, alanine), glycerol, amino acids, propionyl-CoA |
|
|
Glycogen is a polymer of |
glucose |
|
|
what enzyme adds glucose to glycogen |
glycogen synthase |
|
|
What enzyme breaks down glycogen |
glycogen phosphorylase |
|
|
How is glycogen phosphorylase activated |
Glycogen phosphorylase is activated by phosphorylase kinase
Phosphorylase kinase is activated by phosphorylase kinase kinase |
|
|
How are branch points created in glycogenesis |
After 11 glucosyle residues have been added the terminal 6 or 7 are removed as a block. They are moved to a more proximal site on glycogen and attached via alpha 1,6 linkage |
|
|
In which tissues is glucose transport insulin independent |
liver, brain, RBCs |
|
|
In which tissues is glucose transport insulin dependent |
muscle, heart, adipose |
|
|
At what plasma concentration does glucosuria begin to occur |
150 mg/dL |
|
|
These each to what to blood glucose: Insulin Glucagon Epinephrine |
Insulin decreases blood glucose Glucagon increases blood glucose Epinephrine increases blood glucose |
|
|
how do pancreatic beta cells sense blood glucose |
GLUT 2 |
|
|
What are insulin's effects on glucose metabolism |
1. decrease blood glucose 2. stimulate glycolysis in the liver (to make ATP) 3. Stimulates glucokinase activity so glucose can't leave cells (phosphorylated glucose can't diffuse away) 4. inhibits gluconeogenesis in the liver 5. stimulate glycogenesis in the liver and muscle |
|
|
Glycosylation of proteins in DM leads to what complications |
atherosclerosis, retinopathy, nephropathy, neuropathy |
|
|
Treatment for DM: type 1 type 2 |
Type 1: insulin Type 2: diet, exercise, hypoglycemic drugs, insulin |
|
|
What is whipples triad (hint it deals with hypoglycemia) |
Symptoms of hypoglycemia Plasma glucose <45 Amelioration of symptoms by restoration of normal plasma glucose |
|
|
What is the MCC of hypoglycemia |
drug induced |
|
|
Type 0a Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment |
glycogen synthase 2 Liver Hypoglycemia, early death, hyperketonia, low lactate and alanine no treatment |
|
|
Type 1 (von Gierkes) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment (PICMONIC) |
Glucose 6 phosphatase liver increased glycogen leading to severe hypoglycemia and lactic acidosis dietary |
|
|
Type 2 (pompe's) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment (PICMONIC) |
Lysosomal alpha 1 glucosidase all tissue large glycogen vacuols in all cells leading to cardiomegaly and heart failure no treatment |
|
|
Type 3 (cori's) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment (PICMONIC) |
debranching enzyme (alpha 1,6 glucosidase) muscle and liver increased glycogen with short outer branches leading to hepatomegaly with mild hypoglycemia dietary |
|
|
Type 4 (Anderson's) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment |
Branching enzyme Liver and spleen normal glycogen with long filamentous structure leading to hepatomegaly, cirrhosis and death from liver failure no treatment |
|
|
Type 5 (McArdles) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment (PICMONIC) |
Muscle phosphorylase Muscle Exercise intolerance and muscle damage Avoid exercise |
|
|
Type 6 (hers) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment |
Liver phosphorylase liver increase glycogen with normal structure but can have infrequent and mild hypoglycemia dietary |
|
|
Type 7 (Tarui's) Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment |
Muscle phosphofructokinase Muscle Increased glycogen with normal structure. Exercise intolerance unrelieved by glucose Avoid exercise |
|
|
Type 8 Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment |
Liver phosphorylase kinase liver increase glycogen with normal structure. Similar but milder than type 6 (Hers) Dietary |
|
|
Type 9a/b Glycogen storage disease Enzyme deficiency Organ affected Manifestations Treatment |
phosphorylase kinase beta subunit liver, leukocytes, muscle Like Hers because it is pretty much a phosphate deficiency |
|
|
What is the defined level for hypoglycemia |
<45 |
|
|
Describe disorder of fructose metabolism |
patients get fructosuria but the disease is benign. They have fructose intolerance with pain and vomiting if ingested because fructose 1 phosphate builds up and just uses up the intracellular phosphate pool. |
|
|
You need 3 out of 5 of these to Dx metabolic syndrome (give numbers that define each item) |
Central obesity (≥40") HTN (≥130/80) high triglycerides (≥150 fasting) low HDL (<40) insulin resistance (fasting blood glucose ≥100) |
|
|
Weight loss surgery is recommended in what patients |
those with BMI >40 or >35 with co-morbidities |
|
|
Give BMI categories |
underweight <18.5 normal 18.5-24.9 overweight 25-29.9 obesity 30-39.9 extreme obesity ≥40 |
|
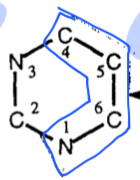
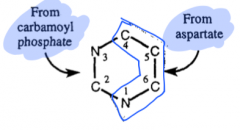
sources of Pyrimidine ring structure |
|
|
|
Control of pyrimidine biosynthesis is accomplished through feedback inhibition of enzymes. What enzymes do the following inhibit: UMP, CMP and UTP |
UMP and CMP inhibit orotidylate decarboxylase UTP inhibits carbamoyl phosphate synthetase II |
|
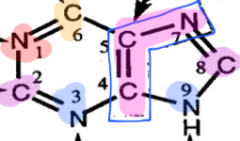
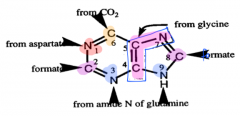
Sources of purine ring structure |
|
|
|
what is the committed step towards purine biosynthesis |
PRPP amino transferase converting PRPP to phosphoribosylamine |
|
|
IMP can become one of two products. What enzymes are used and what are the products |
IMP dehydrogenase makes GMP Adenylosuccinate synthetase makes AMP |
|
|
what form of energy is required to make AMP what form of energy is required to make GMP |
AMP requires GTP hydrolysis GMP requires ATP hydrolysis |
|
|
5-phosphoribosyl 1 pyrophosphate synthetase is inhibited by feedback inhibition of what? |
AMP, GMP, and IMP |
|
|
What is the enzyme that creates deoxyribonucleotides |
ribonucleotide reductase |
|
|
Ribonucleotide reductase requires what to function. What does that require for continued use. |
thioredoxin which requires thioredoxin reductase in order to be recycled for continued use |
|
|
What activates and what deactivates ribonucleotide reductase at the overall activity site |
ATP activates dATP deactivates |
|
|
What does ATP at the specificity site of ribonucleotide reductase favor? |
UDP and CDP reduction to dUDP and dCDP |
|
|
What does dTTP at the specificity site of ribonucleotide reductase favor? |
favors GDP reduction to dGDP |
|
|
What does dGTP at the specificity site of ribonucleotide reductase favor? |
favors ADP reduction to dADP |
|
|
rather than uracil DNA contains what |
Thymine |
|
|
What is the methyl donor for conversion of uracil to thymine |
tetrahydrofolate |
|
|
thymidylate synthetase is inhibited by what drug used as cancer treatment |
5 fluorouracil |
|
|
dihydrofolate reductase is inhibited by what drug used as cancer treatment |
methotrexate |
|
|
What metabolic end product are purine degraded to. What enzyme uses molecular oxygen as part of this breakdown |
uric acid
Xanthine oxidase |
|
|
What are the breakdown products of pyrimidine catabolism and what are the sources |
Uracil is broken down into beta-alanine
Thymine is broken down into beta-aminoisobutyrate |
|
|
Describe the significance of beta-aminoisobutyrate (beta-AIB) |
increased beta-AIB is associated with high DNA diets and cancer chemotherapy so that beta-AIB in the urine correlates with thymidine turnover |
|
|
Orotic aciduria |

|
|
|
Why is orotic acid overproduced by 10x in orotic aciduria |
accumulation of PRPP which is an allosteric activator of carbamoylphosphate syntheses II and the fact that less UTP is produced so there is less feedback inhibition on this same enzyme |
|
|
Lesch-Nyhan Syndrome |

|
|
|
Why is the entire de novo synthesis pathway stimulated in Lesch-Nyhan syndrome |
PRPP is an allosteric activator of the entire pathway
the products of IMP and GMP fall in concentration and therefor their feedback inhibition is lifted. |
|
|
Gout is a disease of altered ___ metabolsim |
purine |
|
|
in HIV what is the function of GP120 |
a major surface protein that binds to T cells through the T cells CD4 and CCR5 |
|
|
which HIV protein pierces the membrane of the target cell and mediates virus fusion |
GP41 |
|
|
How are T cells destroyed in HIV |
lysis of the cells when the replicated virus leaves Immune destruction of cells that express gp120 |
|
|
What is meant by the fact that T cells can form a syncytium if infected with HIV and what is the significance of this |
GP120 can be integrated into the host cell which means it can bind to other T cells through CD4 on the uninfected T cells. Syncytium forming HIV strands tend to be more aggressive and become AIDS faster. |
|
|
what is the inner membrane protein of HIV |
p17 |
|
|
What enzymes are present in HIV |
reverse transcriptase (RNA to DNA) integrase (inserts viral DNA into host genome) protease (cleaves viral proteins out of a long polypeptide) lipids |
|
|
Where would you find lots of HIV virus and where would there be little |
lots in lymphoid tissues because thats where CD4+ T cells are located
Little in the blood |
|
|
What protein is critical for viral replication |
TAT |
|
|
What is the toxicity of TAT to humans |
When TAT is released from infected CD4 T cells it inhibits proliferation of uninfected T cells |
|
|
When is the action of REV |
posttranscriptional |
|
|
What genes are the key infectivity factor. What is its action? |
NEF. It down regulates CD4 receptor level by stimulating internalization of the receptor and prevents superinfection of the cell which would be toxic. |
|
|
What HIV subtype is predominant in north america |
B |
|
|
Why does the HIV virus have high rate of mutation |
Viral DNA polymerase and RNA polymerase lacks error correction |
|
|
What kind of drug is azidothymidine and MOA |
reverse transcriptase inhibitor
Since AZT does not have a free hydroxy group on 3' carbon chain elongation stops and DNA synthesis is aborted. |
|
|
What are the challenges of producing an AIDS vaccine |
they must maintain potent humoral response and induce strong cytotoxic lymphocyte response to be effective |
|
|
Describe the progression of HIV infection |
1-Immune response largely clears the infected cells 2-latent virus escapes detection 3-escaped virus replicates and mutates ahead of the immune system 4-enough immune resistant viral strains exist and stretch the capacity of the immune system 5-immune system deterioration leads to loss of virus replication control |
|
|
1 alpha chain deletion is called |
heterozygous alpha thalassemia 2 |
|
|
two alpha chain deletion on same allele is called |
heterozygous alpha thalassemia 1 |
|
|
two alpha chain deletion on different alleles is called |
homozygous alpha thalassemia 2 |
|
|
3 alpha chain deletion is called? why? |
HbH disease because tetramers of beta chains form hemoglobin H |
|
|
4 alpha chain deletion causes what and produces what type of hemoglobin |
hydrops fetalis
hemoglobin barts (gamma 4) |
|
|
What single amino acid substitution defines sickle cell disease |
The glutamyl residue at position 6 of HbA beta chain is replaced by valine due to a single base change from A to T in the codon for residue 6 of the beta chain making it more hydrophobic on the surface of Hb |
|
|
how does membrane transport change in sickle cell disease |
increase K and Cl cotransport (both leaving the cell) Increased calcium which activates calcium dependent K channels (so K leaves more) Loss of intracellular ions dehydrates the cell |
|
|
How is the membrane altered in sickle cell |
they retain cellular adhesion molecules which can initiate coagulation |
|
|
What is recommended in children with sickle cell |
prophylactic penicillin |
|
|
What is associated with disease progression and death in HIV? how would you counter that? |
Decreased lean body mass
Increase protein intake and activity
Weight loss should be avoided |
|
|
What is higher intake of fruits and veggies associated with |
increased T cell proliferation |
|
|
What can reduce hospitalization and increase CD4 count |
Selenium |
|
|
Supplementation of what reduced immune failure by 4x |
zinc |
|
|
Aside from and HIV patients health status what else should be considered |
Depression |
|
|
Key points in HIV nutrition |
Revisit baseline measurements at each visit
Screen for mental health |
|
|
where does the NADPH required for glutathione reductase come from |
the pentose phosphate pathway |

