![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
100 Cards in this Set
- Front
- Back
|
Describe and explain three types of bonding |
Covalent- Atoms share a pair of electrons in outer shell so both outer shells are full Ionic- Ions with opposite charges have an electrostatic attraction to each other Hydrogen bonding- electrons in molecule not evenly distributed and so spend more time in one place than another so molecule is polar (has uneven distribution of charge). Weak electrostatic bonds formed between polar molecules |
|
|
What makes up polymers? |
Monomer sub-units |
|
|
What is the process of making a polymer called? |
Polymerisation. |
|
|
Give some pollymers made by living organism and their sub-units |
polysaccharides- monosaccharide or single sugar polynucleotides- mononucleotides polypeptides- peptides which are made up of amino acids |
|
|
What is created in organisms each time a new sub-unit is added to a polymer? What is this kind of reaction called? |
Water Condensation reaction |
|
|
what can polymeres be broken down with? What is this kind of reaction called? |
Water Hydrolysis reaction |
|
|
What feature of carbon means it is a building block of living organisms? |
Carbons join to each other to create a back bone along which other elements can join |
|
|
What is a molecule containing carbon called? |
Organic molecule |
|
|
What elements are most biological polymers made from? |
Carbon, hydrogen, oxygen and nitrogen |
|
|
What are the names for a single, double and a long chain of sugars? |
Monosaccharide, disaccharide and polysaccharide |
|
|
Describe monosaccerides |
Sweet tasting, soluble, general formula (CH2O)n where n is any number from 3-7 and it can have isomers |
|
|
Draw the two isomers of glucose |
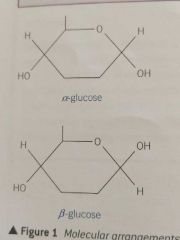
|
|
|
What kind of sugars are reducing? |
All monosaccharides and some disaccharides |
|
|
What is a reducing sugar? |
A sugar which can give electrons to another chemical |
|
|
What is the test for reducing sugars called? Describe it. |
The Benedict's test. Add 2cm3 of food sample to be tested in a tube in liquid or ground in water form Add equal volume of Benedict's reagent Heat mixture for five minutes in gently boiling water bath If reducing sugar present solution turns orange-brown as when Benedict's reagent (alkaline solution of copperII sulphate) is heated with a reducing sugar it forms an insoluble red precipitate of copperI oxide |
|
|
Give the colours of the Benedict's test for different concentrations of reducing sugars |

|
|
|
What are the names of the molecules formed when glucose joins to: Glucose Fructose Galactose |
Maltose sucrose Lactose |
|
|
What is the bond called when monosaccerides join in a condensation reaction? |
Glycosidic |
|
|
Draw out the formation and breaking of a glycosidic bond |
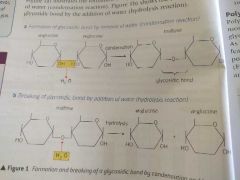
|
|
|
Describe the test for a non-reducing sugar |
Add 2cm3 of the sample to 2cm3 of Benedict's reagent in a test tube and filter it Put test tube in gently boiling water for 5 minutes. If there is no colour change then no reducing sugars are present Add another 2cm3 of food sample to 2cm3 of dilute hydrochloric acid and place in water bath for 5 minutes This will hydrolyse any disaccharides present to monosaccharides Add some sodium hydrogencarbonate solution to a test tube to neutralise acid so benedicts reagent will work. Test pH with pH paper to test solution is alkaline Redo Benedict's test and solution will now turn orange if non-reducing sugars were there in original sample |
|
|
What feature of polysaccharides makes then suitable for storage. What do plants use the polysaccharide cellulose for? |
Insoluble Structural support |
|
|
What form of glucose is starch made from? Where is starch found like in plants? |
Alpha glucose Found in small granules |
|
|
How can you test for starch? |
place 2cm3 of sample in test tube and add a few drops of potassium iodide and shake and it will turn bluey black if starch is present |
|
|
Where is lots of starch found in plants? |
Seeds and storage organs |
|
|
What are two types of chain starch can have |
Branched or unbranched |
|
|
How is the unbranched chain of starch found? What does this mean? |
In tight wound up coils as a helix meaning it is very compact |
|
|
What causes the helix to be formed from starch chains? |
The inward pointing OH group form hydrogen bonds which hold the helix in place |
|
|
What is starches main role and what makes it good at it? |
Energy storage Insoluble so no affect of WP of cell Large so doesn't diffuse out of cell Compact so lots can be stored in a small space Forms alpha glucose when hydrolysed which is easily transported and readily used in respiration Branched form has many ends which many enzymes can act on independently so monomers are released rapidly |
|
|
Is starch in animal cells? |
No |
|
|
What is instead of starch in animal cells? |
Glycogen |
|
|
What are the differences between the structure of glycogen and starch? |
Shorter chains More highly branched |
|
|
Where is glycogen stored in animals? |
As small granules in liver or in muscle cells |
|
|
Why is there little starch in animals? |
Although glycogen stores majority of carbohydrate fat is our main storage molecule |
|
|
Why is glycogen a good storage molecule? |
Insoluble so doesn't effect WP of cell or diffuse out Compact so lots stored in small space Highly branched so rapidly broken down to glucose monomers used for respiration which is needed for animals as they have a higher metabolic and so respiratory rate than plants |
|
|
How does cellulose differ from starch and glucose? |
Made of beta glucose monomers |
|
|
What does the type of glucose cellulose is made from change the structure and function of its pollysaccharide? |
The beta glucose forms straight unbranched chains rather than coils which run parallel to each other so hydrogen bonds can form cross linkages between adjacent chains. Although one hydrogen bond adds little strength many means cellulose is a good structural material |
|
|
How are cellulose molecules arranged and what do these then form? |
Grouped into microfibrils which then for parallel groups called fibres |
|
|
Describe the functions of cellulose in the structure of a plant |
Provides rigidity to plant cells and cellulose cell wall stops cell bursting as water enters by osmosis by exerting inwards pressure to stop any further influx of water This means cells are turgid and push against each other This makes plants semi rigid which helps keep stems and leaves in turgid state giving max surface area |
|
|
What chemical characteristics do all lipids share? |
Contain carbon, oxygen and hydrogen Proportion of oxygen to hydrogen and carbon smaller than in carbohydrates Insoluble in water Soluble in organic solvents such as ethanol |
|
|
What are the two main groups of lipids? |
Triglycerides (fats and oils) and phospholipids |
|
|
Explain some roles of lipids |
contribute to CM flexibility and transfer of lipid soluble products across membrane Source of energy as they provide twice then energy of the same mass of carbohydrate and release water Lipids insoluble in water so used in plants and insects for waxy lipid cuticle which conserve water and mammals produce an oily secretion through sebaceous gland in the skin Fats slow conductors of heat and retain body heat when stored beneath surface Insulate mylein sheath around nerves Protect delicate organs |
|
|
What is the difference between fats and oils at room temp? |
Oils are liquid fats are solid |
|
|
Why are triglycerides called triglycerides? |
They have three fatty acids and each one forms an ester bond with glycerol in a condensation reaction. |
|
|
Draw the formation of a triglyceride |
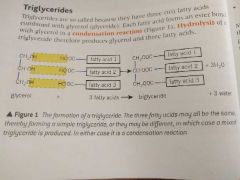
|
|
|
What accounts for the differences in Triglycerides? |
The different fatty acids the glycerol is always the same |
|
|
How many types of fatty acid are there and what do they all have at the end of them? |
70 with COOH group at the end |
|
|
Describe the three types of fatty acid (Carbon bonds) |
Saturated no C=C double bonds Mono-unsaturated one C=C bond Polyunsaturated many C=C bonds |
|
|
Give some properties of triglycerides and how they relate to its structure |
Good source of energy- high ratio of energy storing C-H bonds to carbon atoms Good storage molecules- low mass to energy ratio so lots of energy stored in little volume especially good for moving animals Doesn't affect WP in cells- large non polar so insoluble Good source of water- high hydrogen to oxygen ratio so release water when oxidised |
|
|
What is the difference between lipid and a phospholipid? What effect does this have? |
One of the fatty acid molecules is replaced by a phosphate molecule Phosphate molecules are hydrophillic so a phospholipid has hydrophillic head and hydrophobic tail |
|
|
What are molecules with two ends which behave differently called? |
Polar |
|
|
Draw a phospholipid |
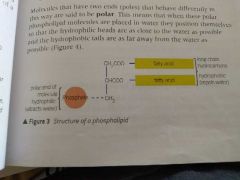
|
|
|
How do phospholipids position themselves in water? |
Heads as close to water as possible and tails as far away as possible |
|
|
Explain some properties of phospholipids |
Molecules form a bilayer in an aqueous environment- due to hydrophobic tail and hydrophilic head e.g hydrophobic bilayer in a cell Form gylcolipids used for cell recognition - by combining with carbohydrates
|
|
|
How would you test for lipids? |
Take dry and grease free test tube Add 5cm3 of ethanol to 2cm3 of sample Shake to dissolve lipid in sample Add 5cm3 of water and shake gently White colour indicates lipid Repeat using water instead of sample as a control |
|
|
Why are dissolved lipids cloudy in water? |
Lipids in the sample are finely dispersed in water and light refracts when passing through emulsion from oil to water droplets |
|
|
What are the monomer units of proteins? |
Amino acids |
|
|
How many amino acids have been identified? How many are found naturally in proteins of all living organisms? What does this prove? |
100 20 Indirect evidence for evolution
|
|
|
Draw an amino acid |
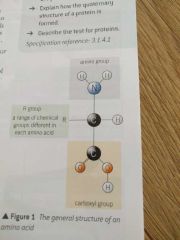
|
|
|
How do two amino acids join? |
Peptide bond from carbon of carboxyl group to nitrogen from amino group of another. Water made from OH of carboxyl group to H of amino group |
|
|
What do single peptides form together? |
Many hundred form polypeptides and the sequence of amino acids in a polypeptide chain forms primary sructure of a protein |
|
|
what determines the sequence of amino acids in a polypeptide chain |
DNA |
|
|
Why are they so many different possible structures of primary proteins? |
100's of the 20 bases joined in in different sequences make a protein so almost limitless combinations |
|
|
What can a change in an amino acid do to a protein? |
Lead to change in shape and stop it working as primary structure determines its ultimate shape |
|
|
Describe how the secondary structure of an alpha helix is formed in a protein |
NH and C=O groups are either side of the peptide bond and the overall positive NH forms a weak hydrogen bond with the overall negative C=O causing the long polypeptide chain to twist into a 3D alpha helix coil |
|
|
What is the tertiary structure of a protein? |
Twisting and folding of alpha helix to give even more complex and often specific 3D shape |
|
|
What decides where bonds in the tertiary structure occur? What are the types of bond that can be in a tertiary structure of a protein? |
Primary structure Disulfide bridge- strong and not easily broken Ionic bonds- between carboxyl and amino groups not in peptide bonds. Weaker than disulfide bridges and easily broken by pH changes Hydrogen bonds- numerous but easily broken |
|
|
What does the 3D structure of a protein allow? |
It allows it to recognise and be recognised by other molecules so it can interact with them specifically due to each protein being distinctive |
|
|
What is the quaternary structure of a protein? |
Can be a number of linked polypeptide chains or non-protein prosthetic groups associated with molecules e.g. iron containing haem group in haemoglobin |
|
|
Describe a test for proteins |
The biuret test which detects peptide bonds Place sample in test tube and add equal volume of sodium hydroxide solution Add few drops of 0.05% copper(II) sulfate solution and mix gently Purple colour indicates peptide bonds are present so there's a protein No proteins if solution stays blue |
|
|
What are the names and roles of the 2 types of proteins? |
Globular-metabolic functions Fibrous- Structural role |
|
|
Describe the structure of fibrous proteins and the primary, secondary, tertiary and quaternary structure of a fibrous protein |
Long chains which run parallel to each other and are linked by cross bridges to form very stable molecules Primary- unbranched polypeptide chain Secondary- Tightly wound chain Tertiary- Chain twisted into second helix Quaternary- three tertiary polypeptide chains wound like rope fibres |
|
|
What conditions must be satisfied for sucrose and water to form glucose and fructose naturally? |
Sucrose ans water molecules must collide with enough energy to to alter arrangement of their atoms to form glucose and fructose Free energy of products must be less than that of substrates Must have activation energy to begin reaction |
|
|
What do enzymes do to activation energies? Why is this good in humans? |
Lower them Metabolic reactions can occur rapidly at normal body temperature |
|
|
Where does a substrate go into on an enzyme and what does this form? |
Into a small depression on enzyme called the active site to form enzyme-substrate complex |
|
|
How is an enzyme held in the active site? |
By temporary bonds formed between certain amino acids of the active site and groups on substrate molecule |
|
|
Describe the induced fit model of enzyme action |
The active site forms as the enzyme and substrate interact. The proximity of the substrate s a change to the enzymes environment and so causes a change in the enzyme which leads to a functional active site forming. The enzyme is flexible and so can mould itself around substrate similar to a glove- general shape stays the same but alters in presence of substrate. As the enzyme changes shape it puts a strain on the substrate which distorts a bond or bonds lowing activation energy for to break bond |
|
|
Describe the lock and key model of enzyme action |
A substrate will only fit the active site of one particular enzyme |
|
|
What supported the lock and key hypothesis? |
Enzymes are specific to reactions they catalyse |
|
|
What are the limitations of the lock and key model? |
The enzyme is considered rigid but other molecules can bind to enzymes at sites other than active site and alter enzymes activity suggesting its shape was altered by molecule. Therefore its structure was not rigid but could be altered |
|
|
What are two changes often observed in enzyme reactions |
Formation of products Reduction of substrate |
|
|
Why do graphs of enzyme catalysed reactions reactions flatten out? |
There are less and less products which means less chance for the products to come into contact with the enzymes and also product might get in the way of substrate and stop it reaching active site Stops when no substrates left so no new product can be produced |
|
|
How do you find rate of change in reaction at any one point? |
Draw a line for the normal then a tangent to the curve then find gradient of that line |
|
|
Describe the shape of the active site compared to the substrate |
Complimentary not the same |
|
|
Why does increasing temperature increase the rate of enzyme catalysed reactions? |
Temperature increase increases KE of molecules so they move more rapidly and the substrates and enzyme will collide more often meaning more effective collisions forming more enzyme substrate complexes and increasing the rate of reaction |
|
|
What can a large temperature increase cause in an enzyme catalysed reaction? |
May cause hydrogen bonds to break in enzyme and change its shape and shape of the active site. Initially at 45C this will cause reaction to slow as substrate fits less easily and at 60C will stop reaction all together |
|
|
What is the name of the process when enzymes no longer work above 60C. How long does this effect the enzyme for? |
Denaturing Permenantly |
|
|
Why is our body temperature 37*C when most enzymes in us function best at 40*C? |
Would speed up reactions but more food would be needed to maintain temperature Other proteins apart from enzymes may denature at higher temperatures At 40*C any further rise e.g. illness may denature enzymes |
|
|
How do you calculate pH? |
-log10(H+ concentration) |
|
|
What effect do pH changes have on enzymes? |
Change reduces rate of enzymes action or if too large denatures enzyme Change alters charges on amino acids which make up active site of enzyme May cause bonds maintaining tertiary structure to break (ionic) Change in H+ ions affects NH2-COOH bond which makes up protein changing shape of active site |
|
|
What effect does enzymes not being used up in reactions have? |
Work even with low enzyme concentration as when it has catalysed one reaction it can do another immediately Can do many substrates a minute |
|
|
What effect does an increase in enzyme concentration have on the rate of reaction? |
Increases it proportionally as long as there is an excess of substrates as there are more substrates than the active sites can cope with so increasing enzyme number means excess substrates can now also be acted upon until all active sites are filed with substrates |
|
|
What effect will an increased substrate concentration have on a reaction? |
If enzyme concentration is constant then the rate of the reaction will increase proportionally to substrate increase until all active sites are full and they are working at full capacity |
|
|
What are enzyme inhibitors are what are the two types? |
Substances which interfere with the functioning of an active site. Can be.... Competitive inhibitors-bind to enzyme's active site Non-competitive inhibitors- bind to enzyme at position other than active site |
|
|
What is a competitive inhibitor and what does it do? |
molecule with a similar shaped to the substrate of an enzyme which allows it to occupy the active site of an enzyme and so it competes with the substrate for available active sites. Has large effect with low substrate concentration and less with a higher one as the inhibitor is not permanently bound so when it leaves another molecule will take its place and how long it takes for a substrate to get in depends on concentration of inhibitor |
|
|
Give an example of a competitive inhibitor |
penicillin to transpeptidase |
|
|
Describe a non competitive inhibitor |
They attach to enzyme at binding site not at the active site which alters the shape of the enzyme and so the active site so that the substrate can no longer occupy it so enzyme is non-functional. |
|
|
What effect does a increase in substrate concentration have on the inhibitor effect for a non-competitive inhibitor? |
Non as substrate and inhibitor are not competing for the same site |
|
|
What is a metabolic pathway? |
A series of reactions in which every step is catalysed by an enzymes laid out in a structured order e.g in a precise sequence on the membrane of a cell organelle inside which the conditions are ideal for the reaction |
|
|
How do chemicals in metabolic pathways help to maintain stable concentration of chemicals in a cell? What is this called? What type of inhibition is end product inhibition normally? |
By using the same chemical as an inhibitor at the start of the metabolic pathway and this is known as end product inhibition Non- competitive |
|
|
What happens if too much of a certain chemical in a metabolic pathway is produced? |
More will create greater inhibition of an enzyme earlier in the pathway and then less of the chemical will be produced so concentration |

