![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Rate law |
Rate = K [A]^x [B]^y K = rate constant |
|
|
Unit of rate constant |
M^(1-n) / time(sec) M = moles per litre n = overall order of reaction |
|
|
How to determine exponents of reactants for rate law |
If rate becomes 2x when conc of A is doubled - order wrt A is 1 If rate becomes 8x when conc of A is doubled - order wrt A is 3 |
|
|
Rate equation for zero order reaction |

|
|
|
Half life of zero order reaction |
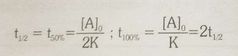
Ao = initial concentration |
|
|
Rate equation for first order reaction |
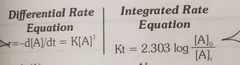
|
|
|
Half life of first order reaction |
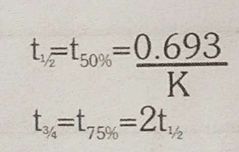
|
|
|
Rate equation for nth order reaction |
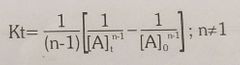
|
|
|
Half life of nth order reacton |
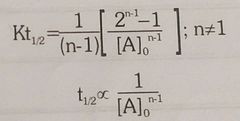
|
|
|
Temperature coefficient |

ratio of the rate constant at two temperatures differ by 10°C, usually from 25°C to 35°C |

