![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
- 3rd side (hint)

|
*Atomic number > tells how many proton there are (as well as electrons) *Atoms of same elements have the same number of protons - so atoms of different elements will have different numbers of protons. |

|
|
|
Compounds are..... CHEMICALLY Bonded! |
* They are formed when atoms of two or more elements are chemically combined together.
Eg, carbon dioxide is a compound formed from a chemical reaction between Carbon & Oxygen. |

|
|
|
ISOTOPES are... |
different atomic forms of the same element, which have the SAME number of PROTONS but a DIFFERENT number of NEUTRONS. |

|
|
|
Ionic Bonding - Transferring Electrons. |
Ionic bonding, atoms lose or gain electrons to form charged particles ( ions) Which are then strongly attracted to one another ( opposite charges + & -) |
|
|
|
Shell with just one Electron is keen to get rid.. |
All left-hand side (of periodic T) atoms, eg, Sodium, potassium...have only one or two outer shell ( highest energy level) So they are keen to get rid of them in order to have full shells ( same as the electronic structure of noble gas) |
So the atoms turn to ions and they leap at the first passing ion with the opposite charge. |
|
|
Nearly full shell is keen to get that Extra Electron... |
On the other hand of PT, (6&7) eg, oxygen and chlorine have outer shells whish are nearly full. *They gain that extra one/ two from other electrons to fill the shell. |

Becoming ions |
|
|
Ionic Compound Have A Regular Lattice Structure |
1) Ionic compound always have giant ionic lattices. 2) The ions form a closely packed regular lattice arrangement. 3) They are very strong electrostatic forces of attraction between oppositely charged ions, in all direction. |

Single crystal of sodium chloride (salt) is one giant ionic lattice, which is why salt crystals are cuboid. |
|
|
Similar properties of Ionic Compounds... |
* All have high melting point & high boiling point due to strong attraction between ions. * Takes large amount of energy to overcome this attraction. * when ionic compound melt , the ions are free to move and they'll carry electric current. |
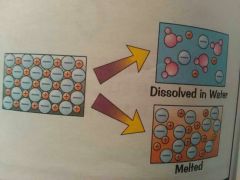
* They dissolve easily in water. The ions separate and are free to move in solution, so they'll carry electrical current. |
|

|

|

|
|

|

|

|
|

|
|

|
|

|

|

|
|

|

|

|
|

|

|

|
|

|

|

|

