![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
What is an ion? |
A charged particle |
|
|
How do you draw an ion? |
As you would a normal atom with square brackets around it |
|
|
Which types of element form ionic bonds? |
Metals with non metals |
|
|
What charge does the metal recieve when it becomes an ion? |
Positive |
|
|
How is a single crystal of sodium chloride arranged? |
In one giant ionic lattice |
|
|
3 properties ionic compounds share and why: |
1. High melting/boiling points due to strong bonds 2. When solid, the ions are held in place so they can't conduct electricity When liquid the ions are free to move so they'll carry an electrical current 3. Dissolve easily |
|
|
Which types of element form covalent bonds? |
Non-metal and non-metal |
|
|
What do covalent bonds do with the electrons? |
Share them |
|
|
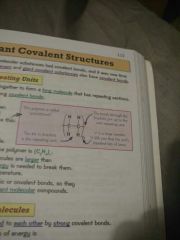
Properties of simple molecular substances and why(4): |
1. Held together by strong covalent bonds, but the forces between molecules is weak 2. Melting / boiling point very low as you only need to break intermolecular forces which are weak 3. Most are gases or liquids at room temperature 4. Can't conduct electricity |
|
What is this a diagram of? |
How to draw polymers |
|
|
How many covalent bonds do each atom in diamond form? |
4 |
|
|
How many covalent bonds does each atom in graphite form? And which shape do they layers form? |
3 and hexagons |
|
|
What element are diamond and graphite made of? |
Carbon |
|
|
What is sand made of? |
Silicon dioxide |
|
|
Why is graphite able to conduct electricity? |
As it has delocalised electrons |
|
|
How many layers of grapite is graphene? |
One. It is only one atom thick |
|
|
What shapes can fullerenes be? |
Spheres or closed tubes |
|
|
What is Buckminster fullerenes molecular formula? |
C60 |
|
|
What can fullerenes be used as and why? |
1. Deliver drugs as they can 'cage' in other molecules 2. Great industrial catalysts ( individual catalysts molecules can be attached) because massive surface area |
|
|
What happens to electrons in metallic bonding? |
They become delocalised |
|
|
Properties of metals and why(2): |
1. Good conductors because free electron 2. Malleable as layers of atoms can slide over each other |
|
|
Why are alloys harder than pure metals? |
Because the different sized atoms distort the shape of the layers so they can't slide over each other |
|

|
:) |

