![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
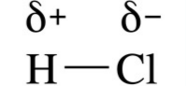
what does this show? |
A permanent dipole (stronger than Van der waals' forces) |
|
|
Ketones have ________ dipole- dipole interactions and __________ points |
lower, boiling |
|
|
The atomic number equals... |
the number of electrons |
|
|
Electrophile |
Reagent attracted to electrons (electron loving) |
|
|
Nucleophiles |
Nuclear loving, donates a pair of electrons in order to form a chemical bond |
|

what is happening here? |
Nucleophilic addition |
|
|
Elements are made up of |
different atoms |
|
|
Number of neutrons and protons in the nucleus is the |
Mass number |
|
|
Removing electrons from an atom creates a positive or negative ion? |
Positive |
|
|
Adding electrons to an atom creates a positive or negative ion? |
Negative |
|
|
Covalent bond |
When two atoms share electrons in order to fill orbitals to make a more stable element e.g. H : O : H |

