![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
127 Cards in this Set
- Front
- Back
|
Which electron has the greatest binding energy?
|
K-shell electrons
|
|
|
What type of electrical charge does the electron carry?
|
Negative charge
|
|
|
Which term describes two or more atoms that are joined by chemical bonds?
|
Molecule
|
|
|
What describes ionization?
|
An atom that loses an electron
|
|
|
The process by which unstable atoms undergo spontaneous disintegration in an effort to attain a more balanced nuclear state is ____?
|
Radioactivity
|
|
|
What are types of particulate radiation?
|
Alpha Particles
Beta Particles Protons Neutrons |
|
|
Nucleons are particulate radiation.
True or False? |
False
|
|
|
Electrons a type of electromagnetic radiation.
True or False? |
False
|
|
|
What are types of electromagnetic radiation?
|
Radar waves
Microwaves X-rays Visible Light Gamma rays Ultraviolet rays Microwaves Radio waves |
|
|
Wavelengths are the distance between ____?
|
Crests of one wave and the next.
|
|
|
X-rays have no charge.
True or false? |
True
|
|
|
X-rays cannot be focused to a point.
True or false? |
True
|
|
|
X-rays cause ionization.
True or false? |
True
|
|
|
X-rays travel at the speed of light.
True or false? |
True
|
|
|
X-rays have more energy than does visible light.
True or false? |
True
|
|
|
What regulates the flow of electrical current to the filament of the x-ray tube?
|
Low-voltage circuit
|
|
|
What is used to increase the voltage in the high-voltage circuit?
|
Step-up transformer
|
|
|
What happens when the high-voltage circuit is activated?
|
Electrons produced at the cathode are accelerated across the tube to the anode.
X-rays travel from the filament to the target. Heat is produced. |
|
|
Where are x-rays produced?
|
At the Positive Anode
|
|
|
Where does thermionic emission occur?
|
At the Negative Cathode
|
|
|
Which accounts for 70% of all the x-ray energy produced at the anode?
|
General Radiation
|
|
|
Which radiation occurs only at 70 kVp or higher and accounts for a very small part of the x-rays produced in the dental x-ray machine?
|
Characteristic Radiation
|
|
|
What is the type of radiation that exits the tubehead?
|
Primary Radiation
|
|
|
What is the type of radiation that has been deflected from its path by interaction with matter?
|
Scatter Radiation
|
|
|
What type of scatter occurs most often with dental x-rays?
|
Compton Scatter Radiation
|
|
|
Physics is defined as ____?
|
The study of the relationship between matter and energy.
|
|
|
Matter is anything that occupies___?
|
Space, has mass, and has inertia.
|
|
|
Definition of Inertia:
|
The ability of objects to remain at rest if at rest. or to move if moving, unless affected by some outside force.
|
|
|
Matter may be found in 3 forms:
|
1. Solid
2. Liquid 3. Plasma |
|
|
Energy is ?
|
The potential, or ability to do work.
|
|
|
What results when matter is altered?
|
Energy
|
|
|
Different forms of Energy:
|
1. Mechanical
2. Electrical 3. Chemical 4. Heat 5. Light 6. Radiation Energy |
|
|
Law of Conservation of Energy?
|
Energy can neither be created nor destroyed.
|
|
|
Energy can be converted to other forms.
True or false? |
True
|
|
|
________ is the study of those aspects of physics pertaining to the origin, nature and behavior of x-rays and related types of radiation?
|
Radiation Physics
|
|
|
_____ is a beam of energy that has the power to penetrate substances and record image shadows on photographic film.
|
X-ray
|
|
|
_____ is a form of energy carried by waves or a stream of particles.
|
Radiation
|
|
|
What are the seven shells of an atom, in order of binding energy.
|
K, L, M, N, O, P, Q
|
|
|
___ keeps electrons in their orbit.
|
Electrostatic force
|
|
|
Another word for electrostatic force?
|
Binding energy
|
|
|
______ Radiation travels in straight lines at high speeds.
|
Particulate radiation
|
|
|
_____ radiation transmits kinetic energy and heat.
|
Particulate radiation
|
|
|
What are the 4 kinds of Particulate Radiation?
|
1. Electrons
2. Alpha particles 3. Protons 4. Neutrons |
|
|
What are the 2 types of Electrons of Particulate Radiation?
|
1. Beta particles
2. Cathode Rays |
|
|
What are Beta Particles?
|
Fast moving electrons emitted from the nucleus of radioactive atoms.
|
|
|
What are cathode rays?
|
Streams of fast electrons that originate in x-ray tube.
|
|
|
What are Electrons of Particulate Radiation?
|
They are classified as beta particles and cathode rays.
|
|
|
What are Alpha particles of the Particulate Radiation?
|
Emitted from nuclei of heavy metals, exist as two protons and neutrons, without electrons.
|
|
|
What are Protons of the Particulate Radiation?
|
Accelerated particles of hydrogen nuclei with mass of 1 and a charge of +1.
|
|
|
What are Neutrons of the Particulate Radiation?
|
Accelerated particle with a mass of 1 and no electrical charge.
|
|
|
____ is the production of ions, or the process of converting an atom into ions.
|
Ionization
|
|
|
Deals with electrons ONLY!
|
Ionization
|
|
|
Ionizing radiation defined?
|
Radiation that is capable of producing ions by removing or adding an electron to an atom.
|
|
|
What is High-Energy Ionizing Radiation?
|
X-radiation that is used in diagnostic imaging.
|
|
|
The emission and propagation of energy through space/substance in forms of waves.
|
Radiation
|
|
|
A process of spontaneous disintegration of unstable atom in an effort to attain a more balanced nuclear state.
|
Radioactivity
|
|
|
Two groups of Ionizing Radiation:
|
1. Particulate Radiation
2. Electromagnetic Radiation |
|
|
What is Particulate Radiation?
|
Tiny particles of matter that possess mass.
|
|
|
What is Electromagnetic Radiation?
|
No mass, wave-like energy
|
|
|
Man-made wave-like energy that goes through space or matter. Has no mass.
|
Electromagnetic Radiation
|
|
|
X-rays:
|
1. Form of energy
2. Belongs to the Electromagnetic Radiation Group. 3. Measured in short and long wave lengths. |
|
|
_____ are bundles of pure energy, no weight, no mass, & travel at the Speed of LIGHT. AKA Photons.
|
X-rays
|
|
|
_____ travel through space as particle and in wave form.
|
Photons
|
|
|
Wavelengths are?
|
Distance between crests.
|
|
|
The ____ the distance, the ____ the wavelength, the ____ the energy to penetrate matter.
|
Shorter, Shorter, Higher
|
|
|
kVp settings affect ___ ?
|
Radiation power.
|
|
|
The number of times the wavelength passes in certain amount of time.
|
Frequency
|
|
|
____ gives us the number of tiems we have crest in a wave.
|
Frequency
|
|
|
If Frequency is high, then wavelength is _____ ?
If Frequency is low, then wavelength is ____ ? |
Short
Long |
|
|
Characteristics of Long Waves:
|
Low Frequency
Less Energy Less Penetrating |
|
|
Characteristics of Short Waves:
|
Higher Frequency
More Energy More Penetrating |
|
|
X-rays are ____ waves, ____ energy & _____ frequency. They are _____ penetrating.
|
Short
High High More |
|
|
Properties of X-Rays:
|

|
|
|
X-ray Appearance:
|
Invisible and cannot be detected by any of the senses.
|
|
|
X-ray Mass:
|
No mass or weight.
|
|
|
X-ray Charge:
|
No charge.
|
|
|
X-ray Speed:
|
Travel at the speed of light.
|
|
|
X-ray Wavelength:
|
Travel in waves, short wavelengths with high frequency.
|
|
|
X-ray Path of Travel:
|
Straight lines, can be deflected or scattered.
|
|
|
X-ray Focusing Capability:
|
Cannot focus to a point.
Always diverge from a point. |
|
|
X-ray Penetrating Power:
|
Can penetrate liquids, solids, gases.
The composition of the substance determines whether x-rays penetrate or pass through, or are absorbed. |
|
|
X-ray Absorption:
|
Absorbed by matter which depends on the atomic structure of matter & the wavelength of the x-ray.
|
|
|
X-ray Ionization Capability:
|
Interact with materials they penetrate and cause ionization.
|
|
|
X-ray Fluorescence Capability:
|
Can cause certain substances to fluoresce or emit radiation in longer wavelengths.
ie: Visible light, Ultraviolet light |
|
|
X-ray Effect on Film:
|
Can produce an image on photographic film.
|
|
|
X-ray Effect on Living Tissues:
|
Causes biological changes in living cells.
|
|
|
Three component parts of an X-ray machine.
|
1. Control Panel
2. Extension Arm 3. Tubehead |
|
|
What parts does the Control Panel Contain?
|
1. On/Off Switch
2. Indicator Light 3. Control Devices (kVp & mA selector) |
|
|
What is in the Extension arm?
|
House wires to all the components.
|
|
|
What are the components of the Tubehead?
(Hint: 13) |
Metal housing
Insulating oil Tubehead seal X-ray tube Transformer Aluminum Discs Lead Collimator PID (position indicating device) Step Up & Step Down Transformers |
|
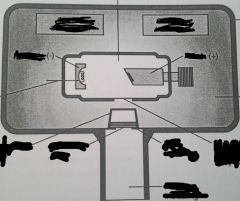
Name the Tubehead Component Parts
|
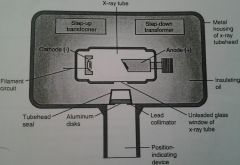
|
|
|
What are the components of the X-ray Tubehead?
|
1. Metal housing of tubehead
2. Insulating Oil 3. Tubehead Seal 4. X-ray Tube 5. Transformer 6. Aluminum Disks 7. Lead Collimator 8. PID (Position-Indicating Device) AKA Cone |
|
|
X-ray Tubehead:
Metal Housing |
1. Metal housing
2. Surrounds the x-ray tube & transformers. 3. Filled with oil 4. Protects the x-ray tube & grounds the high-voltage components. |
|
|
X-ray Tubehead:
Insulating Oil |
Oil that absorbs the head generated from the x-rays and prevents overheating.
|
|
|
X-ray Tubehead:
Tubehead Seal |
1. Either aluminum or leaded-glass covering of the stubehead that permits the exit of x-rays from the tubehead.
2. Seals the oil in the tubehead & acts as a filter to the x-ray beam. |
|
|
X-ray Tubehead:
X-ray Tube |
Heart of the x-ray generating system.
|
|
|
X-ray Tubehead:
Transformer |
1. Device that alters the voltage of incoming electricity.
2. Either Step-Up or Step-Down |
|
|
X-ray Tubehead:
Aluminum Disks |
Sheets of 0.5 mm thick aluminum placed in the path of the x-rays that is used as a filter to block out the low penetrating long wavelength x-rays.
|
|
|
X-ray Tubehead:
Lead Collimator |
1. Lead plate with a central hole that fits directly over the opening of the metal housing, where the x-ray exits.
2. Restricts the size of the X-ray. |
|
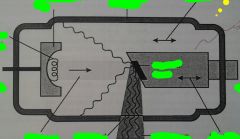
What are the Components of the X-ray Tube?
|
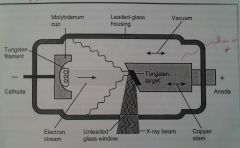
Changes To My Labial Vagina Are Truely Cosmetic, Xpect Ultrahot Environment.
|
|
|
X-ray Tube:
Catholde |
1. Negative Electrode
2. Consists of the Tungsten Filament in the cup-shaped holder made of molybdenum. 3. Purpose: Supply the electrons necessary to generate x-rays. 4. Electrons are accelerated toward the positive anode. |
|
|
X-ray Tube:
Tungsten Filament |
1. Coiled wire made of tungsten, which produces electrons when heated.
2. Located in the Cathode. |
|
|
X-ray Tube:
Molybdenum Cup |
1. Focuses on the electrons into a narrow beam and directs the beam across the tube toward the tungsten target of the anode.
2. Located in the Cathode. |
|
|
X-ray Tube:
Leaded-Glass Housing |
Leaded-glass vacuum tube that prevents x-rays from escaping in all directions.
|
|
|
X-ray Tube:
Vacuum |
Space surrounding the insides of the x-ray tube.
|
|
|
X-ray Tube:
Anode |
1. Positive electrode
2. Consists of wafer thin tungsten plate embedded in a solid copper rod. 3. Purpose: To convert electrons into x-ray photons. 4. Includes the Tungsten target and the Copper stem. |
|
|
X-ray Tube:
Copper Stem |
Functions to dissipate the heat away from the tungsten target.
|
|
|
X-ray Tube:
X-ray Beam |
Consists of short and long wavelengths.
The aluminum disks filter out long wave lengths and leave the short wavelengths. |
|
|
X-ray Tube:
Unleaded Glass Window |
1. This allows the x-ray beam to exit the tube.
2. Directs the x-ray beam toward the aluminum disks, collimator, and PID. |
|
|
X-ray Tube:
Electron Stream |
Moves from the cathode to the anode. Anode changes electrons to photons.
|
|
|
X-ray Tube:
Tungsten Target |
Plate of tungsten that serves as a focal spot & converts bombarding electrons into x-ray photons.
|
|
|
mA Amperage
|
mA controls the number of electrons passing through the cathode filament.
|
|
|
mA Amperage
|
Determines the X-ray beam QUANITY (amount of electrons passing thru the cathode filament).
|
|
|
mA
Amperage |
1. Controls heating
2. Hotter filament more electrons 3. Controls Cloud of Electrons 4. Has to do with the NUMBER of X-rays produced. |
|
|
Kilovoltage
|
1. Controls the QUALITY, penetrating ability, of the x-ray beam.
|
|
|
kVp
Kilovoltage Peak |
1. Controls the voltage (power) of the current passing from cathode to anode.
2. Quality of x-rays 3. Affects speed of electrons hitting Tungsten Target. |
|
|
Transformers:
Step Up Step Down |
1. Used to increase or decrease the voltage in the electrical circuit.
2. Located in tubehead |
|
|
General Radiation
|
When the electron passes close to the nucleus of a tungsten atom is slowed down, this energy is produced.
|
|
|
Characteristic Radiation
|
When an electron dislodged an inner-shell electron from the tungsten atom resulting in the arrangement of the remaining orbiting electrons and the production of an x-ray photon known as _______.
|
|
|
Definition of X-radiation:
Primary Radiation |
1. The penetrating x-ray beam tha tis produced at the target of the anode & that exits the tubehead.
2. AKA primary beam or useful beam. |
|
|
The penetrating x-ray beam that is produced at the target of the anode and that exits the tubehead.
|
Primary Radiation
|
|
|
X-radiation that is created when the primary beam interacts with matter. Less penetrating than the primary radiation.
|
Secondary Radiation
|
|
|
A form of secondary radiation that is the result of an x-ray that has been deflected from its path by the interaction with matter.
|
Scatter Radiation
|
|
|
No Interaction of X-Radiation
|
the x-ray photon passes thru the atom unchanged and leaves the atom unchanged.
|
|
|
Photoelectric Effect of X-Radiation
|
Accounts for 30% of radiation between x-ray and matter.
|
|
|
Coherent Scatter of X-Radiation
|
When a low energy x-ray photon interacts with an outer shell electron there is no loss of energy and no ionization.
|
|
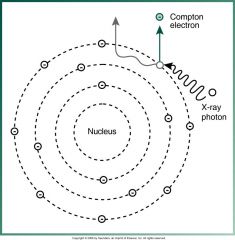
What type of Scatter Radiation is this?
|
Compton Scatter
|

