![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
96 Cards in this Set
- Front
- Back
|
Define the following: matter |
anything that takes up space and has mass |
|
|
Define the following: density |
degree of consistency measured by the quantity of mass per unit volume |
|
|
Define the following: proton |
subatomic particle positive charge +1 1 amu P+ |
|
|
Define the following: neutron |
subatomic particle neutral charge 0 1 amu N |
|
|
Define the following: electron |
subatomic particle negative charge -1 0 amu e- |
|
|
Define the following: homogeneous |
a material that has the same properties throughtout |
|
|
Define the following: Chemistry |
the science of substances, their structure, properties, and the changes they undergo |
|
|
Define the following: physical change |
things you can observe without changing what it is |
|
|
Define the following: chemical change |
a substance's ability to undergo changes that transform it in to another thing |
|
|
Define the following: photon |
a particle of light that carries a quantum of energy |
|
|
Define the following: energy level |
fixed amount of energy that a molecule can have in it's outer shell |
|
|
Define the following: excited state |
atom absorbs energy |
|
|
Define the following: ground state |
atom gives off energy |
|
|
Define the following: noble gases |
group 18 of elements |
|
|
Define the following: plum pudding model |
model of the atom J. J Thomson |
|
|
Define the following: spin quantum number |
S two possible spin states clockwise/counter-clockwise |
|
|
Define the following: angular/orbital quantum number |
L (cursive) subshell or shape s, p, d, f |
|
|
Define the following: continous spectrum |
all the colors of the rainbow |
|
|
Define the following: dark-line spectra |
absorption of light in a particular wavelength |
|
|
Define the following: bright-line spectra |
everything is absorbed except a few specific wavelengths |
|
|
Define the following: isotope |
atoms of a chemical element whose nuclei have the same atomic number but have different masses |
|
|
Define the following: ion |
an atom with a net eletrical charge due to loss/gain of electrons |
|
|
Define the following: lone pair |
pair of valence electrons that are not shared |
|
|
Define the following: octect rule |
each element can only have 8 valence electrons it their outer shell except H & He only 2 |
|
|
Define the following: optical isomers |
two or more forms of a compound mirror image |
|
|
Define the following: diatomic molecules |
HON & the Halogens compounds that form naturally in space |
|
|
Define the following: qualitative observations |
non-numerical |
|
|
Define the following: qauntitative oberservations |
numerical |
|
|
Define the following: chemical bond |
how atoms gain/lose electrons sharing: covalent take/give: ionic |
|
|
Define the following: structural isomers |
the atoms are completely arranged in a different order |
|
|
Define the following: lewis dot structures |
shorthand way to show number of valence electrons |
|
|
Define the following: metallic bonds |
sea of electrons delocalized: the electrons fly around all over high melting & boling point soft good conductors lustrous/malleable/ductile |
|
|
Define the following: electronegativity |
ability of an atom to attract electrons to itself in a chemical bond |
|
|
Define the following: subscript |
how many atoms are in the formula |
|
|
Define the following: superscript |
charge or oxidation number +1 1+ |
|
|
Define the following: coefficent |
how many molecules |
|
|
Define the following: ionic compounds |
electrons held tightly together magnetism holds it together |
|
|
Define the following: covalent compounds |
nonmetal and nonmetal bonding |
|
|
Define the following: multivalent |
elements with 2 or more possible charges +1 only = Ag +1, +2 = Cu, Hg +2, +3 = Cr, Ni, Fe, Co +2, +4 = Pb, Sn |
|
|
Define the following: empirical formula |
gives lowest whole number ratio of the atoms in the compound |
|
|
Define the following: molecular formula |
shows the actual number and kinds of atoms present in compounsd |
|
|
Define the following: cation |
positive ion |
|
|
Define the following: anion |
negative ion |
|
|
Define the following: alloy |
a metal made by combining 2 or more metallic elements |
|
|
Define the following: atomic number |
number of protons |
|
|
Define the following: atomic mass |
mass in atoms expressed in Atomic Mass Units |
|
|
What is the SI base unit for time? |
seconds |
|
|
What is the SI base unit for mass? |
grams |
|
|
What is the SI base unit for length |
meter |
|
|
What is the SI base unit for volume |
liter |
|
|
What is the SI base unit for temperature |
Celsius |
|
|
What are the 6 branches of chemistry, and their definitions? |
1. organic- compounds containing Carbon 2. inorganic- compounds not containing Carbon 3. biochemistry- chemistry of life 4. theoretical chemistry- chemical theory 5. analytical chemistry- sample chemistry 6. physical chemistry- physics chemistry |
|
|
What are the five states of matter and their properties? |
1. liquid: no fixed shape; fixed volume 2. solid: fixed shape; fixed volume 3. gas: no fixed shape; no fixed volume 4. plasma: consists of highly charged particles with extremely high kinetic energy 5. bose-einstein condensate: close to absolute zero; almost no kinetic energy atoms clump together |
|
|
Elements with the same propertiese are located in? |
groups |
|
|
Convert 8.0 cg into kg |
0.00008 kg |
|
|
What is the density of a substance if a 5.00 ml samples weighs 96.5 g? |
m/v 96.5g/5.00mL 19.3 g/mL |
|
|
How do you convert Celsius into Fahrenheit and vise versa? |
Celsius = 5/9(F--32) Fahrenheit = (9/5 * C) + 32 |
|
|
Lewis Structure for: SiF4 |

|
|
|
Lewis Structure for: MgCl2 |
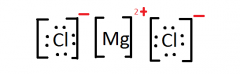
|
|
|
Lewis Structure for: PF3 |
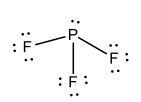
|
|
|
Acids typically start with which element? |
Hydrogen |
|
|
Calculate the moles present in: 2.00 g of H2O |
.111 moles |
|
|
Calculate the moles present in: 75.57 g of KBr |
1.708 moles |
|
|
Calculate the moles present in: 100. g of KClO4 |
.722 moles |
|
|
Calculate the moles present in: 8.76 g of NaOH |
.219 moles |
|
|
Calculate the moles present in: 0.750 g of Na2CO3 |
.00707 moles |
|
|
Calculate the grams present in: 0.200 moles of H2S |
6.82 g |
|
|
Calculate the grams present in: 0.100 moles of KI |
16.6 g |
|
|
Calculate the grams present in: 1.500 moles of KClO3 |
183.8 g |
|
|
Calculate the grams present in: 0.750 moles of NaOH |
30.0 g |
|
|
Calculate the grams present in: 3.40 x 10^-5 moles of Na2CO3 |
.004 g |
|
|
What is the trend in Atomic Radii? |
size of atom increases as you go left and down Fr is the most He is the least |
|
|
What is the trend in reactivity? |
increases as you go left to right |
|
|
What is the trend in electronegativity? |
increasese up and right not including the noble gases |
|
|
What is the percent compostition of Potassium Hydroxide? |
K = 97.5% H = 2.5% |
|
|
What is the percent composition of Mercury (II) Nitrate? |
Hg = 61.8% N = 8.6% O = 29.6% |
|
|
Write the formula for the following: Tin (IV) Peroxide |
Sn2(O2)4 |
|
|
Write the formula for the following: Silver Cyanide |
Ag(CN) |
|
|
Write the formula for the following: Magnesium Phosphide |
Mg3P |
|
|
Write the formula for the following: Carbon Monoxide |
CO |
|
|
Write the formula for the following: Trinitrogen Heptahydride |
N3H7 |
|
|
Write the formula for the following: Dihydrogen Monoxide |
H2O |
|
|
Name the following: Cr3(PO4)2 |
Chromium (II) Phosphate |
|
|
Name the following: CO2 |
Carbon Dioxide |
|
|
Name the following: HNO3 |
Nitric Acid |
|
|
Name the following: Fe2(SO4)3 |
Iron Sulfate |
|
|
Name the following: C6H7N |
Hexacarbon Heptahydrogen Mononitride |
|
|
Name the following: Al2O3 |
Aluminum Oxide |
|
|
A CSI chemical analysist is trying to determine the cause of a fire. She knows through tests that the molar mass of the substance is 138.55g. After seperating out a 46.17g sample; its found to contain 11.82g of chlorine, 13.03g of potassium, and 21.33g of oxygen. What is the name of this chemical? |
ClKO4 |
|
|
Convert 4.87 cups to mL |
1152.185 |
|
|
Name the acid: HCl |
Hydrochloric Acid |
|
|
Name the acid: HNO3 |
Nitric Acid |
|
|
Name the acid: H2SO4 |
Sulfric Acid |
|
|
Name the acid:H3PO4 |
Phosphoric Acid |
|
|
Name the acid: HC2H3O2 |
Acetic Acid |
|
|
Name the acid: H2CO3 |
Carbonic Acid |

