![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
117 Cards in this Set
- Front
- Back
|
function of kidney
|
- regulation of volumes and osmolarities of fluids
- regulation of ion concentrations and pH - excretion of wastes and foreign substances (decontamination) - production of hormones (renin, erythropoetin, prostaglandins) |
|
|
The urinary system: anatomical highlights
|
Kidneys position is retroperitoneal, i.e. outside and behind the abdominal cavity
Each kidney is connected to the aorta and vena cava with short renal artery and vein, posing essentially no hydrodynamic resistance The urine flows out of the kidneys via ureters into the bladder From the bladder urine is discharged via the urethra |
|
|
kidney picture
|
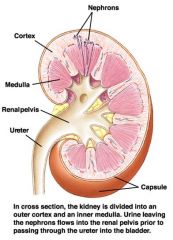
|
|
|
how many arterioles and and capillaries are associated with each nephron
|
2
the afferent and efferent arteriole and the glomerulus capillaries and peritubular capillaries |
|
|
excretion equals what?
|
F-R+S=E
|
|
|
total water reabsorption?
|
20 percent of fluid filters.....greater than 19% of this is reabsorbed
|
|
|
GBM proteins? ex
|
type 4 collagen
laminin entactin |
|
|
peptidoglycans ex
|
chondroitin sulfate proteoglycans
and heparin |
|
|
NE effect on kidney
|
afferent arteriole has more A1 receptors...constricts this diverting blood elsewhere
also NE + Epi act on b1 receptors in juxtaglomerular cells, increasing secretion of renin (which increases levels of Angiotensin II) |
|
|
Angiotensin 2 effect on kidney
|
constricts efferent arterioles
NE + Epi act on b1 receptors in juxtaglomerular cells, increasing secretion of renin (which increases levels of Angiotensin II) |
|
|
sympathetic stimulation effect on GFR and RBF
|
The separate effects of NE and AgII represent a purely experimental situation, in reality, under sympathetic stimulation, they work together and the net effect is decreased GFR and RBF
|
|
|
exercise effect on RBF and GRF
|
Sympathetic stimulation and exercise reduce both the renal blood flow (RBF) and glomerular filtration rate (GFR)
|
|
|
Prostaglandins on RBF and GFR
|
Prostaglandins increase RBF and GFR
|
|
|
ACh on GFR
|
ACh typically causes renal vasodilation and increases GFR (parasympathetic input to kidneys arrives via the vagus nerve)
|
|
|
autoregulation mechanisms
|
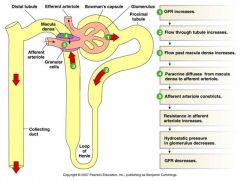
Autoregulation mechanisms:
1. Myogenic response = the intrinsic ability of smooth muscle to contract in response to increased tension in the vascular wall (more pronounced upon pressure increase) ...when it is stretched, ion channels open and the cell depolarizes causing it to contract 2. Tubuloglomerular feedback = paracrine effect from the macula densa on the afferent arteriole in response to increased or decreased GFR (ATP, adenosine and NO are currently being studied as paracrines) Macula densa also induces renin secretion from juxtaglomerular (JG) cells when GFR is low |
|
|
what is the kidney flow meter?
|
the juxtoglomerular apparatus
|
|
|
where are most precious substances reabsorbed?
|
Most of the “precious” substances (sugars, amino acids) are reabsorbed in the proximal tubule (PCT).
Most of the actively secreted substances also leave the blood into PCT |
|
|
where do most actively secreted substances leave the blood?
|
at the PCT
|
|
|
renal threshold
|
the point when the amount of filtered substance exceeds the reabsorption capacity of the nephron
F>R |
|
|
substances reabsorbed with sodium
|
water
Na+, H2O and many substances are reabsorbed in PCT Reabsorption of many substances is coupled to Na+ transport and is driven by Na+ gradient : D-glucose, D-galactose (via Na+/glucose cotransporter, SGLT) Amino acids (via 5 different types of symporters for neutral, basic and acidic amino acids) Inorganic anions PO33-, SO42- are taken up via Na+- coupled transporters Npt2 and NaS1, respectively |
|
|
epithelial sodium blocker
|
amiloride (potent diuretic)
|
|
|
sodium transport from tubule lumen to intersitiial fluid
|

channel is called ENaC: epithelial sodium channel
it can be blocked by amiloride |
|
|
close homolog of ENaC channel?
|
ASIC-1
|
|
|
sodium/glucose cotransporter
|

SGLT
Basolateral side: glucose transport facilitator |
|
|
how does hydrogen secretion occur?
|
H+ secretion is coupled to Na+ uptake via the Na+/H+ exchanger
All of the following leave blood by ABC (atp binding cassetes) transporter... Hydroxybenzoates Hippurates (paraamino hippurate, PAH) Catecholamines (neurotransmitters) Bile pigments Antibiotics, toxins, drugs |
|
|
renal threshold for glucose
|
300mg/100ml
if exceeded, glucose appears in the urine |
|
|
talk about diabetes mellitus
|
Diabetes mellitus - a metabolic disorder characterized by abnormally high blood concentration of glucose. Urine is sweet (diabetes mellitus = honey-urine disease).
“fasting blood sugar test” : the norm is <120 mg/dL Type 1 (insulin-dependent, juvenile) - insufficient insulin production (pancreatic b cells do not respond to the elevated level of glucose in the plasma). Insulin injection decreases the level of glucose because tissues respond normally. Type 2 (NIDDM, adult-type) - levels of insulin are normal or even above the norm, but insulin-sensitive cells do not respond (suppressed receptor/pathway or the effector protein GLUT4). Insulin injection does little to the blood glucose level. |
|
|
control of insulin/glucose level by B-cells in the pancreas
|

purple is glucose
grean is insulin: glucose absorption by tissues increased ATP leads to blockage of K+ sulfonylurea receptor leading to membrane depolarization |
|
|
What happens in response to insulin
|

green is insulin
purple is Glucose arrows are glut4 In response to insulin, GLUT4 is incorporated into the plasma membrane (in many tissues, but most of all in muscles and liver) GLUT4 - glucose diffusion facilitator 1) Glucose enters pancreatic B cells 2) Increased Atp 3) causes K+ channel to close 4)calcium rushes in 5) insulin released 6) insulin causes Glut-4 to be incorporated in muscle and liver cells. |
|
|
talk about insulin and receptor
|
Insulin is a dipeptide
(21 and 30 amino acids) interlinked by three S-S bonds Isulin Receptor belongs to the Receptor Tyrosine Kinase family, requiring intracellular adapter proteins |
|
|
symptoms and progression of type 1 diabetes
|
The glucose level hits the threshold - glucosuria
less water is reabsorbed by kidney - osmotic diuresis Polydypsia - constant drinking as a compensation for fluid loss If compensation fails - then dehydration (hypovolumea) occurs, insufficient tissue perfusion, hypoxia and switch to glycolytic metabolism. This causes metabolic acidosis Polyphagia - excessive eating often accompanied by weight loss The cells can not receive glucose, they ‘think’ they are starving. Glycogen breakdown, gluconeogenesis from amino acids, and beta-oxidation of fatty acids are turned on. The latter causes ketoacidosis and ketourea Kidney suffers when the capacity of excreting H+ is exceeded (this aggravates acidosis) The combination of metabolic acidosis (+ ketoacidosis) and hypoxia from circulatory collapse causes coma. Treatment: insulin replacement + fluid and electrolyte therapy. High plasma glucose downregulates glucose transport across the blood brain barrier. This results in hypoglycemia in the brain, especially after insulin injection. It may be severe enough to cause coma and even death. |
|
|
transport in nephron of water and other stuff
|

PCT sodium is pumped out and h20 follows
descending limb is passive ascending limb is active Lecture 35 3a |
|
|
draw countercurrent exchange mechanism
|
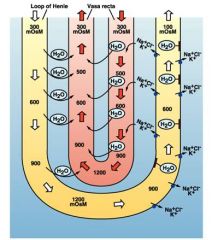
f
|
|
|
vasopressin release and curve
|

Vasopressin release is controlled by the osmosensory neurons in hypothalamus. It is released when plasma osmolarity raises above 280 mOsm
Vasopressin release is inhibited when BV and venous pressure are above normal (negative feedback from sensory neurons in atria) |
|
|
difference between vasopressin and oxytocin
|
difference in two amino acids
|
|
|
vasopressin abest vs present
|
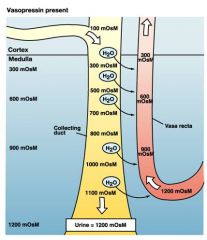
absent water channels are close and the MOsM stays at 100
|
|
|
types of aquaporins
|

24 kDa polypeptide. Functional complex is a tetramer.
AQP-2 – apical, ADH dependent. AQP-4 – basolateral, constitutively present (ADH independent) structure ensures strict water orientation in the channel that excludes any proton wire and ion transport |
|
|
water balance input and output.
|
Input: Food and drink ~2.1 L/day
Metabolism 0.3 L/day Output: Evaporation through skin and lungs 0.7 L/day Urine 1.5 L/day Feces 0.2 L/day |
|
|
mug example of kidney
|

a
|
|
|
all about vasopresin
|

Vasopressin (antidiuretic hormone, ADH)
- Family of 9 amino acid peptides (with one disulfide bond between cysteines 1 and 6, aminated on the carboxy terminus). AVP (Arginine vasopressin) is the most abundant C-Y-F-Q-N-C-P-R-G Receptors and pathways: V1 receptor in vascular walls (G-PLC-IP3), mediates the vascular pressor response V2 receptor in the collecting duct (G-cAMP-PKA) mediates the antidiuretic action in the collecting duct Synthesis of vasopressin takes place in the Hypothalamus, it is released from the posterior pituitary Vasopressin release is controlled by the osmosensory neurons in hypothalamus. It is released when plasma osmolarity raises above 280 mOsm Vasopressin release is inhibited when BV and venous pressure are above normal (negative feedback from sensory neurons in atria) |
|
|
what detects osmosensory?
draw pathway |

SON (supraoptic nucleus in the hypothalamus
|
|
|
3 things ADH effects
|
plasma osmolarity
blood volume blood pressure |
|
|
diseases associated with disorders in aquaporins
|
Diabetes insipidus
cataracts brain edema |
|
|
diabetes insipidus overview
|
Diabetes insipidus
passage of large amount of dilute urine (polyurea) drinking of large amount of water (polydipsia) The general cause is inability of kidney to react to vasopressin 1. vasopressin deficiency due to brain disease, tumors, lesions or trauma 2.nephrogenic form is caused by congenital defects either in the V2 receptor gene (X-linked), or in the AQP2 gene (autosomal) Diagnostics: AQP2 normally occurs in urine. In vasopressin deficiency there is a prompt raise of AQP2 in response to a vasopressin agonist. There is no raise in AQP2 in the urine in either of the nephrogenic forms. |
|
|
homeostatic respnose to salt ingestion
|
L33 pg 3c
|
|
|
Blood volume regulation by kidney
|
L33 pg3d
|
|
|
control of renin secretion diagram
|

JG cells are also called juxtaglomerular cells or granular cells and are attached on the afferent arteriole
|
|
|
RAAS pathway
|
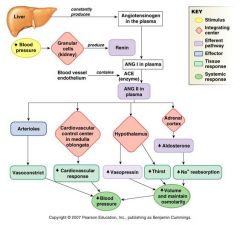
renin angiotensin aldosterone system
|
|
|
talk about angiotensin receptor and the different pathways
|
Angiotensin receptor = AT1 (G-protein coupled)
- via the PLC-IP3 pathway it induces: Vasoconstriction (BP, TPR ) Aldosterone synthesis Facilitation of neurotransmission, augments effects of NE - via the PLA2 and PLD pathways it activates synthesis of prostaglandins synthesis of growth factors and extracellular matrix proteins, leading to cardiac hypertrophy and remodeling of the cardiovascular system. |
|
|
ANP and ANP pathway
|

Atrial Natriuretic Peptide (ANP)
28 amino acids peptide, a member of growing family of NP Released by the atrial endocardium in response to distension ANP has short life time in circulation (minutes) ANP receptor is coupled to Guanilyl Cyclase 1) Reduces Na+ and water reabsorption (blocks Na/K pump) 2)Increases GFR by relaxing afferent arteriole and mesangial cells 3)Inhibits release of renin and therefore production of angiotensin and aldosterone 4)Inhibits vasopressin and reduces activity of the presser area in the medulla oblongata |
|
|
Perturbations of volume and osmolarity picture
|
lecture 33 pg 1c
|
|
|
concentrations of substances in the nephron and reason why?
|
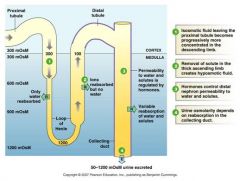
a
|
|
|
fast and slow responses to hypovolumea diagram
|
lecture 34 pg1b
|
|
|
dehydration flow chart
|
lecture 34 2b
|
|
|
talk about thirst reaction
|
lecture 34 2c
|
|
|
Ka
describe buffer draw a buffer diagram |
lecture 34 2d
|
|
|
pH
|
-log [h+]
|
|
|
henderson-hasselblach eqn
|
ph= pka + long [anion]/[ha]
|
|
|
fixed acids and bases of the body
|
lecture 34...3b
|
|
|
bicarbonate is a what?
|
volatile buffer
|
|
|
normal range for plasma ph
|
7.38-7.42
|
|
|
Normal [HCO3-] in plasma
|
23-26 mM
|
|
|
urine ph ranges
|
4.5 to 8.5
|
|
|
Decrease in plasme pH =
|
acidosis
Neurons are less excitable → depression → coma |
|
|
Increase in plasma pH
|
alkalosis
overexcitation → tetanus → block of respiration |
|
|
H2CO3 is proportional to what
|
the pressure of CO2
|
|
|
bicarbonate buffer equation
|
CO2 + H2O <> H2CO3 <> H+ + HCO3-
|
|
|
metabolic acidosis is caused by
|
diarrhea (loss of bicarbonate)
renal failure (insufficient H+ secretion) diabetes mellitus (ketoacidosis) |
|
|
respiratory acidosis caused by
|
lung disease (obstructive, asthma)
injury to the respiratory control ctr |
|
|
Metabolic alkalosis
caused by? |
vomiting (loss of gastric acid)
|
|
|
Respiratory alkalosis
|
hyperventilation, anxiety
|
|
|
respiratory acidosis compensation diagram
|
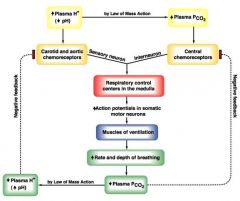
caused by chemoreceptors
|
|
|
Davenport diagram
use discussion picture...also explain all stuff in book and be able to explain |

CO2 + H20 = H2CO3 = H+ + HCO3-
|
|
|
DRAW TUBULE WITH
WHERE EVERYTHING IS SECRETED AND REABSORBED EVERYTHING!!!! |
35 pg3A
|
|
|
renal threshold curve
|
30 4a
|
|
|
handling of various substances by kidney
|
30 5c
|
|
|
general mechanisms of renal compensation for acidosis
|
35 3b
|
|
|
constitutive H+ secretion and HCO3- reabsorption
|

in pct
1) sodium hyrdrogen antiport secretes hyrdrogen 2) h in filtrate combines with filtered bicarb to form co2 3) co2 diffuses into cell and combines w water to form h and bicarb 4) h is secreted again and excreted 5) bicarb is reabsorbed 6) glutamine is metabolized to ammonium ion and bicarb 7) ammonium is secreted and excreted 8) bicarb is reabsorbed |
|
|
what do intercalated cells do and describe
|

Intercalated cells in the collecting duct secrete or reabsorb H+ and HCO3- according to the needs of the body
|
|
|
specialized transporters
|
in PCT:
Na+/H+ Exchanger Na+/NH4+ Exchanger (NH4+ transport is coupled to amino acid breakdown) in intercalated cells in DCT: H+/K+ ATPase H+ ATPase HCO3-/Cl- Exchanger (Band3-like) |
|
|
plasma pco2, ions, ph in acid-base disturbances
|

metabolic alkalosis hco3- should be up
|
|
|
renal compensation for respiratory deviations and vice versa
what causes it...compensation? |
lecture 36 1d
|
|
|
normal sodium concentration in plasma/ecf is?
|
135-145 mM
|
|
|
ingest how much nacl?
|
9g /day
|
|
|
body does what with regards to sodium conc? kidney?
|
monitors sodium conc and kidney excretes excess
|
|
|
is sodium permeable through plasma membrane?
|
no and because of this it is the major extracellular osmolyte
|
|
|
do small changes in sodium conc out result in dramatic changes of its reversal potential?
|
no
|
|
|
talk about high and low salt and potassium
|
Hyponartemia = excess water or loss of Na+
hypoosmotic dehydration may decrease the extracellular fluid volume (due to vomiting, diarrhea, overuse of diuretics, or sodium-wasting kidney diseases) Typically associated with lower blood pressure. Hypernatremia = increased plasma [Na+] and osmolarity. Hyperosmotic overhydration due to excessive secretion of aldosterone causes higher BP. Hyperosmotic dehydration (more water lost than Na+) occurs in diabetes insipidus Unlike Na+, the extracellular concentration of K+ critically influences EK and the resting potential. Deviations of [K+] upset excitable tissues (nerves and muscles) Normal [K+]out = 3.5-5 mM In hypokalemia ([K+]out < 2.5 mM) resting potential is lower (more negative), tissues are less excitable. Muscle weakness becomes dangerous when it effects the respiratory muscles and the heart. Can be corrected by K+-rich food. In hyperkalemia ([K+]out > 6 mM) nerves and muscles become initially more excitable, then loose excitability. The first symptom is cardiac arrhythmia as the resting potential gets close to the threshold. |
|
|
cells involved in sodium, potassium, and calcium transport
|
Principal (P) cells in DCT and cortical CD are involved in Na+, K+ and Ca2+ transport
|
|
|
aldosterone pway
|
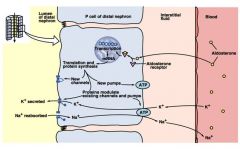
The response of principal cells in DCT to aldosterone
Aldosterone regulates Na+ and K+ concentrations reciprocally: higher reabsorption of Na+ is coupled to faster secretion of K+ k+ secretion is ROMK na+ reabsorbed is ENaK |
|
|
basics of calcium
concentrations functions |
Normal [Ca2+]pl = 2.5 mM
Ca2+ is an intracellular signal, second messenger [Ca2+]cytoplasm = 10-6 - 10-7 M, more in organelles - major chemical component of bones - it is critical for contractility of all muscles - cell signaling - it is involved in synaptic transmission & secretion (exocytosis) - serves as “cement” for tight junctions and extracellular adhesion molecules - co-factor in blood coagulation and many other processes |
|
|
low vs high calcium levels
what is retention of calcium accompanied by? |
Hypocalcemia typically leads to hyperexcitability
Under low [Ca2+]out voltage-gated channels (Na+, Ca2+) do not gate properly. The threshold potential is lower. Hypercalcemia causes depression of neuromuscular activity Plasma [Ca2+] is closely monitored by Ca2+ receptors (G-protein coupled) in thyroid and parathyroid glands Retention of Ca2+ is always accompanied with excretion of phosphate |
|
|
most common form of ca storage in the body
|
hydroxyapatite....also major component of bones
|
|
|
total Calcium
|
99% bones
0.1% ecf intracellular 0.9%...mostly in ER, SR, and mitochondria |
|
|
draw bone growth
|

1)osteoblasts lay bone on top of cartlidge
2)old chondrocytes disintegrate 3)chondrocytes produce cartlidge 4)dividing chondrocytes add length to bone |
|
|
what do osteoclasts do?
|

secrete acid and enzymes to dissolve the bone
|
|
|
total balance of calcium diagram
|

23-21
|
|
|
PTH
|
84 amino acid peptide. Released in response to a decrease in plasma [Ca2+].
Acts on the G-protein coupled PTH receptors present in effector cells in kidney, bone, intestine PTH indirectly stimulates osteoclasts, which increase bone resorption. This mobilizes Ca2+ from bone. PTH enhances renal reabsorption of Ca2+ in the distal nephron. Simultaneously it reduces phosphate reabsorption (increases secretion) PTH increases intestinal absorption of Ca2+ by increasing synthesis of vitamin D |
|
|
vitamin d
|
also known as calcitrol
Steroid, formed by sunlight acting on precursor molecules, then converted in two steps (in liver and kidney) to 1,25(OH)2D3 Stimulated by reduced plasma [Ca2+], via PTH or prolactin Acts on nuclear receptors Stimulates production of calbindin, a Ca2+ binding protein which accomplishes intestinal transport of Ca2+ (by unknown mechanism) Mn: 1)Letter D sunbathing in sun is activated 2)When cows don't fan D (calcitrol: controls cows), it becomes stimulated via PTH or prolactin 3)when it is stimulated it creates a nuclear weapon (acts on nuclear receptors) 4)allows something to bind to cows so they do they do their job again (calbindin) 5)this allows for the cow to be ate by the intestine. |
|
|
calcitonin
|
Released from C cells of thyroid gland in response to elevated plasma [Ca2+]
Acts via a G-protein coupled receptor Target organs: bone and kidney Prevents bone resorption (when osteoclasts break bone down into its minerals and release it into the plasma), enhances kidney excretion of Ca2+ Stabilizes abnormal bone loss during pregnancy or in osteoporosis |
|
|
Osteoporosis
what can reduce risk? what can reduce resorption? |
Abnormal loss of bone mass due to decreased bone dep(step..brick)osition and/or increased rate of bone resorption
Bones become brittle The disease has a genetic component, affects more women, comes with age, depends on habits and diet. Estrogen replacement therapy at postmenopausal age reduces the risk Ca2+-rich diet and weight–bearing exercise preserve bone mass Special studies show that mechanical stress applied to bones reduces the rate of resorption. Weightlessness (space flight condition) increases resorption. |
|
|
name the major transporters
|
in PCT:……………………..
ENaC (epithelial Na+ channel), SGLT (Na+/glucose cotransporter) Na+/amino acid co-transporters Na+/H+ Exchanger, K channels Na+/NH4+ Exchanger (NH4+ transport is coupled to amino acid breakdown) in DCT:…………….. H+/K+ ATPase H+ ATPase HCO3-/Cl- Exchanger (Band3-like), Na+/K+/2Cl- cotransporter, Na/Cl electroneutral uptake system Epithelial Na+ (ENaC) and K+ (ROMK) channels |
|
|
diuretics (name 5)
|
Water - inhibits Vasopressin (ADH) secretion
Xantines (caffeine, theophylline) – decrease tubular reabsorption of Na+ and increase GFR Carbonic anhydrase inhibitors (reduce Na+ reabsorption through reduction of Na+/H+ exchange). Amiloride (Colectril) – blocks ENaC channels Furosemide (Lasix) - loop diuretic, inhibits Na+/K+/2Cl- cotransporter Thiazide – acting on DCT, blocks electroneutral Na/Cl co-transport, also leads to increased loss of K+ simply due to higher flow. Mercurial compounds – block aquaporins Hormone/receptor inhibitors: Ethanol - inhibits Vasopressin (ADH) secretion Antagonists of the V2 vasopressin receptor (peptide and non-peptide) cure hyponatremia Spironolactone – is an antagonist of aldosterone receptor. Decreases both Na+ reabsorption and K+ secretion at the same time. Is a moderate potassium-sparing diuretic. |
|
|
talk about micturition
|

Micturition = desire to urinate, voiding reflex
Bladder may contain up to 500 ml of urine. It empties through a single tube called urethra. Urethra is closed by two muscular rings or sphincters. Internal sphincter is made of smooth muscle. Normal tone keeps it contracted External sphincter is a skeletal muscle ring controlled by motor neurons and higher brain centers, so it is under conscious voluntarily control. Simple micturition reflex takes place in infants. After the stage of toilet-training, the simple micturition reflex is usually inhibited until the person consciously desires to urinate. Higher CNS centers inhibit parasympathetic signals that lead to bladder contraction. Inflammations result in higher sensitivity to internal pressure. ‘Bashful bladder’ – inability to urinate under certain circumstances. |
|
|
kidney diseases
|
Gout, Kidney stones – results of insufficient excretion/higher reabsorption of uric acid. Accumulation and precipitation of uric acid in tissues or in the renal pelvis. Gout has uric acid in tissue
Mn: your stones are ucky and goooooeeey. Proteinurea (albuminurea) – increased permeability of the glomerular filtration membrane to proteins. When the loss of protein into urine exceed the production by the liver, this condition may lead to edema Uremia – accumulation of toxic waste products of protein metabolism in the blood. Correlates with increased levels of urea and creatinine. Symptoms: lethargy, anorexia, vomiting, confusion, mental deterioration, muscle twitching, convulsions, coma. Uremia can be treated with HEMODIALYDIS. Polycystic kidney disease: cross section of pyramids have fluid filled bubbles; bulging points in nephron....polarity of epithelial is incorrect A total kidney failure may require a kidney transplant. ...humans can still survive w/ 1/8th kidney capacity |
|
|
draw a hemodialysis
|

f
|
|
|
fistulla
|

used in permanent dialysis patients
|
|
|
fistula
|
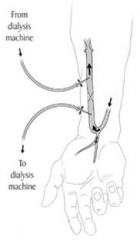
in patients who need dialysis regularly, a clamp in the vein is put.
|
|
|
water
|
inhibits vasopressin secretion
|
|
|
xantines
|
caffeine, theophylline
decrease tubular reabsorption of sodium and increase GFR |
|
|
carbonic anhydrase inhibitors
|
reduce sodium reabsorption through reduction of sodium/hydrogen exchange
MN: CA SH |
|
|
amiloride
|
blocks ENaC
amber riding in an epithelial car filled with salt |
|
|
furosemide
|
lasix
loop diuretic, inhibits sodium/potassium/chloride co-transporter Mn: furry coat prevents sodium, potassium, and chloride from leaving in the loop |
|
|
thiazide
|
acting on dct, blocks electroneutral na/cl co-transport, also leads to increased loss of K+ simply due to higher flow
Mn: picture the z as being special in that it is a sodium /chloride co transporter |
|
|
mercurial compounds
|
block aquaporins
Mn: picture a thermometer being stuck into a plug that water should go through |
|
|
ethanol
|
inhibits vasopressin (ADH) secretion
|
|
|
spironolactone
|
antagonist of aldosterone receptor. decreases both sodium reabsorption and potassium secretion at the same time. Is a moderate potassium-sparing diuretic
Mn: arnold was a spy and on roids |

