![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
What are the primary inputs and outputs of H+ of the body to maintain a serum pH balance?
|
"Inputs:
1) Diet - Fatty acids, amino acids 2) Metabolism - CO2 (+H2O), lactic acid, ketoacids Outputs: 1) Ventilation - CO2 (+H2O) 2) Kidneys - H+" |
|
|
By what mechanism does vomiting change serum pH? Diarrhea?
|
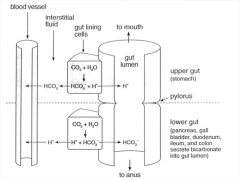
Loss of hydrogen ions from the stomach through vomiting, forcing the stomach to produce more acid, thus raising serum bicarbonate levels (alkalosis).
Diarrhea causes a loss of bicarbonate, as the bowel is generally rich in bicarbonate to neutralize the stomach acid. This leads to acidosis. |
|
|
What is the major and most commn ECF buffer?
|
Bicarbonate - HCO3
|
|
|
What does the pK indicate on a titration curve of a bufffering agent?
|
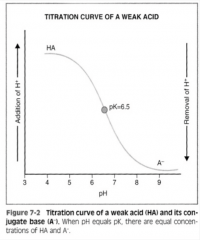
An equal concentration of hydrogen donor and hydrogen acceptor.
|
|
|
What is the Henderson-Hasselbach equation?
|
!["In the case of bicarbonate:
pH = pK + log [HCO3-]/[0.03pCO2], where pK = 6.1"](https://images.cram.com/images/upload-flashcards/61/92/35/2619235_m.png)
In the case of bicarbonate:
pH = pK + log [HCO3-]/[0.03pCO2], where pK = 6.1 |
|
|
What does the ratio have to be between [HCO3] and [CO2] have to be to maintain a pH of 7.4?
|
20:1
Because: pH = 6.1 + log 20 = 7.4 |
|
|
What percentage of filtered bicarbonate is reabsorbed by the kidney?
|
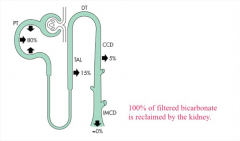
100%. If you see bicarbonate in the urine, there is a defect in renal transport.
|
|
|
How is bicarbonate reabsorbed by the proximal tubules and the Loop of Henle of the kidney?
|
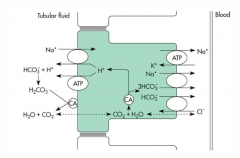
Indirectly, through the actions of the Na+ - H+ exchanger.
1) Hydrogen ions are secreted and react with the filtered bicarbonate. 2) Carbonic acid is then formed rapidly, which reacts with carbonic anhydrase located on the surface of a apical membrane. The carbonic acid quickly disassociates into water and CO2. 3) The CO2 then diffuses into the cell, where it combines with water to form carbonic acid once again. 4) The interior of the cell is rich in carbonic anhydrase, forming bicarbonate and a hydrogen ion. 5) The hydrogen ion is recycled and the bicarbonate ion is transported out into the bloodstream. |
|
|
How is bicarbonate reabsorbed by the distal nephron of the kidney?
|
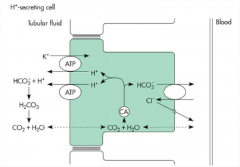
Indirectly via a hydrogen-ATPase pump
1) Hydrogen ions are secreted and react with the filtered bicarbonate. 2) Carbonic acid is then formed rapidly, which reacts with carbonic anhydrase located on the surface of a apical membrane. The carbonic acid quickly disassociates into water and CO2. 3) The CO2 then diffuses into the cell, where it combines with water to form carbonic acid once again. 4) The interior of the cell is rich in carbonic anhydrase, forming bicarbonate and a hydrogen ion. 5) The hydrogen ion is recycled and the bicarbonate ion is transported out into the bloodstream. |
|
|
How does the kidney use urinary buffers to generate bicarbonate?
|
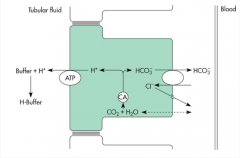
Occurs in the distal portion of the nephron.
1) Bicarbonate is produced within the cells through the dissociation of carbonic acid. 2) The hydrogen ion is pumped out of the cell at the apical membrane by the ATPase pump, where it combines with a urinary buffer (phosphate, sulfate). 3) The bicarbonate intracellulary is transported out, restoring depleted stores of bicarbonate." |
|
|
How does the kidney utilize ammonium (NH4+) to generate new bicarbonate?
|
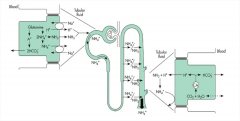
"1) The proximal tubule begins by metabolizing filtered glutamine, producing bicarbonate and ammonia.
2) The bicarbonate is transported into the bloodstream, while the ammonia is transported into the urine compartment via the sodium - hydrogen exchanger." |
|
|
A change in __________ (bicarbonate/carbon dioxide) levels will primarily lead to respiratory acid base disorders.
|
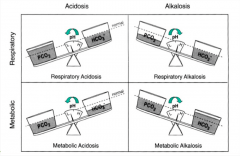
Carbon dioxide
|
|
|
A change in __________ (bicarbonate/carbon dioxide) levels will primarily lead to metabolic acid base disorders.
|
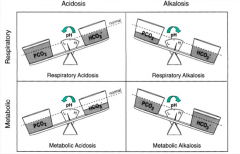
Bicarbonate.
|
|
|
for primary metabolic disorders, which way does the pH and bicarb move with respect to each other?
|
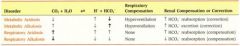
same direction
|
|
|
for primary respiratory disorders, which way does the pH and bicarb move with respect to each other?
|
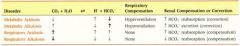
opposite direction
|

