![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
109 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are the five cardinal signs of inflammation? What causes them?
|
1. Rubor
- dilatation of small blood vessels 2. Calor - increased blood flow / chemical mediators 3. Tumor - oedema i.e. fluid in the extracellular space 4. Dolor - stretching of tissues due to oedema / presence of chemical mediators 5. Functio Laesa - pain / swelling |
|
|
|
What are the four typical causes of a 'red hot joint'?
|
>Infection
>Crystal deposition >'Flare' in chronic arthritis >Trauma |
|
|
|
What are the initial changes to blood vessels during acute inflammation?
|
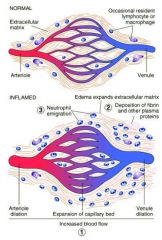
1. Vessel diameter increases - capillaries
2. Increased capillary hydrostatic pressure and escape of plasma proteins into the extravascular space 3. Decreased colloid osmotic pressure draws less fluid back into vessels 4. Protein rich fluid accumulates outside the vessels = EXUDATE |
|
|
|
What is the arachidonic acid pathway and its role in inflammation?
|
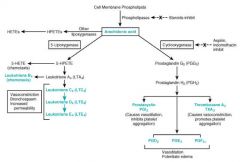
>Arachidonic acid is a precursor in the production of eicosanoids:
- The enzymes cyclooxygenase and peroxidase lead to prostaglandin H2, which in turn is used to produce the prostaglandins, prostacyclin, and thromboxanes. - The enzyme 5-lipoxygenase leads to 5-HPETE, which in turn is used to produce the leukotrienes. >Arachidonic acid is also used in the biosynthesis of anandamide. >Some arachidonic acid is converted into hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) by epoxygenase.[7] >The production of these derivatives and their action in the body are collectively known as the "arachidonic acid cascade" ROLE: >Key inflammatory intermediate and vasodilator |
|
|
|
Name two causes of increased vascular permeability.
|
1. Severe direct vessel injury (sustained)
2. Chemical mediators - Histamine, bradykinin, NO, C5a, LTB4, PAF (immediate) |
|
|
|
Name a delayed cause of increased vascular permeability.
|
>Endothelial cell injury (e.g. by bacterial endotoxins - prolonged)
|
|
|
|
What is the role of neutrophils in inflammation?
|
1. Adhesion to microorganisms
2. Phagocytosis 3. Intracellular killing of organisms |
|
|
|
What are the main mediators of acute inflammation?
|
>Vasoactive amines - histamine, serotonin
>Complement components >Eicosanoids - arachidonate >Clotting cascade >Kinin cascade |
|
|
|
What are the beneficial effects of acute inflammation?
|
>Dilution of toxins
>Entry of antibodies and drugs >Fibrin formation >Cell nutrition and oxygenation >Start of immune response |
|
|
|
What are the harmful effects of acute inflammation?
|
>Damage to normal tissues via
- enzymic digestion - abscess cavities - vascular damage >Swelling in critical sites (epiglottis, brain) >Immune hypersensitivity reactions - apparently inappropriate response causing tissue damage and even life threatening consequences e.g. asthma |
|
|
|
What are outcomes of the red hot joint? Possible systemic effects?
|
OUTCOMES:
>Resolution >Suppuration and severe damage to the joint >Spread to nearby bone (osteomyelitis) SYSTEMIC EFFECTS: >Pyexia >Malaise, anorexia, nausea >Lymphoreticular hyperplasia >Raised ESR, raised WCC |
|
|
|
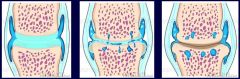
What may occur to an inflamed joint?
|
>Acute inflammation with the presence of neutrophil polymorphonuclear leucocytes causes damage to cartilage - irreversible
>May eventually reach underlying bone and completely destroy the joint in some kinds of infection of not treated early and effectively >Gout and RA are more chronic processes which have acute flares, both can destroy the joint and cause loss of bone from the edge of the joint |
|
|
|
What is the structure of synovium?
|
>Synovial surface is villous (increases surface area)
>Contains: - Type A cells: Macrophages containing lysosomal enzymes - Type B cells: secrete hyaluronic acid |
|
|
|
What are the functions of synovium?
|
>Control of diffusion in and out of joint
>Ingestion of debris >Secretion of hyaluronate, immunoglobulins, and lysosomal enzymes >Lubrication of the joint >Provide nourishment for the avascular articular cartilage |
|
|
|
What are the constituents of articular cartilage?
|
>Collagen (90% type II)
>Proteoglycans >Chondrocytes - protein synthesis - phagocytosis >70-80% water by weight >Damage to articular cartilage can be serious and irreversible |
|
|
|
What factors effect joint mobility?
|
>Joint mobility depends on lubricity of cartilage surfaces bathed in synovial fluid
>Cartilage function depends on proteoglycan (which is hydrophilic) contained by collagen fibres >Changes in proteoglycan and collagen result in loss of water and therefore of resilience |
|
|
|
Name 3 characteristics of primary osteoarthritis.
|
>Sometimes familial - intrinsic defect of the joint or its cartilage
>Incidence increases with age (wear and tear) >More common in females >Affects multiply joints bilaterally >Heberden's nodes (osteophytes of the distal interphalangeal joints) |
|
|
|
What is the pathogenesis of OA?
|
>In osteoarthritis, cartilage is lost from points of contact
>Increased load - exercise and obesity - make degenerative disease worse >Most of the load on joints is absorbed by the muscles >Decreased resilience of cartilage due to cross-linking with ageing |
|
|
|
What are 3 characteristics of cartilage pathology?
|
>Flaking - loss of proteoglycan
>Fibrillation >Eburnation - marbling |
|
|
|
What pathological changes occur to bone as a consequence of OA?
|
>Thickening of subchondral trabeculae
>Grooving of bone surface >Cracking of bone admits synovial fluid → subchondral cystic spaces >Osteophyte formation (Heberden's nodes) |
|
|
|
What are the signs and symptoms of OA?
|
>Pain relieved by rest
>Stiffness >Crepitus on movement >Limitation of movement >Compression of spinal nerve roots |
|
|
|
What is secondary OA and what may it be caused by?
|
>Degenerative changes in joints previously damaged by some other disease process
>Diseases which may lead to secondary degenerative changes include: - septic arthritis - tuberculosis arthritis - rheumatoid arthritis - gout - congenital dislocation of the hip |
|
|
|
What are the causes of OA?
|
CAT IN BED
Congenital dislocation of the hip Acquired: Traumatic - fracture Infective Neoplasm Blood / biochemical (gout, haematochromatosis) Endocrine - diabetes mellitus (peripheral neuropathy) Degenerative - overuse |
|
|
|
What is RA?
|
>A systemic chronic inflammatory disease
>Can occur at any age but prevalence increases with age - M:F 1:3 >Variable course: remissions and exacerbations >Probably a heterogeneous group of disorders |
|
|
|
What is this?
|
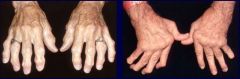
Osteoarthritis -- Rheumatoid arthritis
Note characteristic rheumatoid nodules and the MCP joints affected causing severe ulnar deviation of the digits. |
|
|
|
What is the pathogenesis of RA?
|
>Genetic predisposition (HLA Dw4)
>Autoimmunity - Rheumatoid factor is multiple antibodies to the Fc fragment of IgG >Cell mediated immunity - T lymphocytes in synovium >?Infectious agents (EBV or other viruses) |
|
|
|
What pathology is found in RA?
|
>Synovial inflammation - pannus
>Erosion of adjacent bone >Joint destruction and fibrous fusion >Systemic - rheumatoid nodules - vasculitis |
|
|
|
What is Perthe's disease?
|
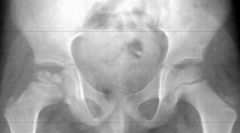
Perthes' disease occurs in a part of the hip joint called the femoral head. This is the rounded top of the femur (the thigh bone) which sits inside the acetabulum (the hip socket). Something happens to the small blood vessels which supply the femoral head with blood. So, parts of the femoral head lose their blood supply. As a result, the bone cells in the affected area die, the bone softens, and the bone can fracture or become distorted. The severity of the condition can vary.
Over several months the blood vessels regrow, and the blood supply returns to the bone tissue. New bone tissue is laid down, and so the femoral head reforms and regrows. This is similar to how bone reforms and regrows after any normal fracture or break to a bone. But, with Perthes' disease, it takes longer (up to several years). The main concern with regrowth of the femoral head is to ensure that it forms a good spherical (rounded) shape. This helps it to fit well into the hip joint socket. If the femoral head is less rounded, hip movements may be affected and there may be more wear and tear on the hip joint. |
|
|
|
Name two organisms which typically infect bone.
|
>Staphylococcus Aureus
>Mycobacterium Tuberculosis |
|
|
|
Name two organisms which typically infect joints.
|
>Staphylococcus Aureus
>Staphylococcus Epidermidis (Prostheses) >Various Anaerobes |
|
|
|
Name two organisms which typically infect muscle.
|
>Clostridium Perfringens
>Streptococcus Pyogenes |
|
|
|
"
What are three typical routes to contracting an infection of the bone? |
> Haematogenous osteomyelitis in which bacteria reach bone through the circulation
> Traumatic osteomyelitis, particularly following road accidents, war injuries, wounds, fractures or surgery, in which bacteria gain access to bone > Spread from an adjacent infected joint |
|
|
|
Which class of bacteria are typically responsible for osteomyelitis?
|
>Staphylococci are responsible in about 75% of cases
>However, if osteomyelitis complicates sickle-cell disease, or some other haemoglobinopathy, there may be different causative organisms, particularly Salmonella spp. >In neonates, streptococci (group B) or enterobacteria e.g. Escherichia coli may be the infecting organism >Infection can also be with M. tuberculosis – if in the spine it causes vertebral collapse – Pott’s Disease |
|
|
|
How does acute haematogenous osteomyelitis present itself?
|
This is a surgical emergency.
>The cardinal signs are of acute inflammation - swelling, pain, redness and loss of function. >It is the supreme example of the axiom 'Where there is pus let it out' – before it causes pressure necrosis of the bone. - Do so with the least possible delay. >Laboratory findings include leucocytosis in 50% and elevated acute phase reactants |
|
|
|
Describe the localisation and pathology of acute haematogenous osteomyelitis.
|
>The metaphysis of a long bone is the usual site but any bone can be involved, sometimes several at once
- Infection thromboses the end arteries of a metaphysis, and so kills the bone that they supply - Pus accumulates under pressure, breaks out through a hole in the bone, and comes to lie under the periosteum - Pus then strips the periosteum off the shaft and deprives part of the bone of its blood supply, so that it dies and forms a sequestrum - The stripped periosteum responds by producing new bone, which is the beginning of the involucrum - Later, this may become so extensive that it forms a new shaft >If the disease progresses, large areas of bone, and perhaps even its entire shaft, become separated from their blood supply, die, and form one or more sequestra - These lie inside the involucrum, bathed in a pool of pus, which discharges through sinuses in the skin >Occasionally, the disease does not go through this acute stage, and does not form sequestra, or sinuses - Instead, the infected bone becomes sclerotic, and its marrow cavity is obliterated (Brodie's abscess) >Before the age of six months an epiphysis offers no barrier to the spread of infection, so that pus in a metaphysis rapidly spreads to a joint - After this age the cartilage of an epiphyseal plate limits the spread of infection, so that a joint is only infected if an infected metaphysis extends inside a joint capsule, as in the proximal femur |
|
|
|
What is the treatment for acute haematogenous osteomyelitis?
|
>If acute – drain the pus – send specimens to microbiology
>Give Flucloxacillin + Fucidin empirically >Give antibiotics of appropriate sensitivity if infecting organism is NOT a Staphylococcus or if resistant to Flucloxacillin /Fucidin >Treatment should be for at least 6 weeks |
|
|
|
How does TB osteomyelitis present?
|
>The patient may present with an unexplained fever, a fracture or neurological signs caused by collapse of a vertebra.
|
|
|
|
What is the pathology of TB osteomyelitis?
|
>Mycobacteria enter the body by the lungs where they are taken up by alveolar macrophages where they replicate
- Mycobacteria are transported to all parts of the body within the circulating macrophages - Their cell wall has a complex structure and includes lipo-arabinomannan (LAM) – the equivalent of the lipopolysaccharide of Gram- negative bacteria - LAM has important biological activity and inhibits macrophage activation, induction of cytokine release and suppression of T cell activation >The characteristic histological feature is the granuloma. |
|
|
|
What is the treatment for TB osteomyelitis?
|
>Treatment should be for at least 6 months and is normally 9 months for bone infections
>Combination therapy is used to minimise emergence of drug resistance: Rifampicin + isoniazid + pyrazinamide + ethambutol (3-4 months), then rifampicin + isoniazid (up to 9 months) >Treatment can be reduced if combined with radical surgery >Bed rest and bracing may be beneficial in cases of spinal TB - Bracing may arrest curve progression and provide pain relief |
|
|
|
Which organisms typically cause acute tissue infections?
|
>Streptococcus pyogenes (Group A strep)
>Mixed infections with facultative and strict anaerobes >Clostridium perfringens |
|
|
|
What are the clinical features of gas gangrene?
|
Clinical presentation – gas gangrene:
>There is usually a history of surgery, or trauma >There is acute onset of severe localised pain and hypotension >Clostridium perfringens is a Gram-positive anaerobic rod-shaped bacterium that produces numerous exotoxins - It can form very resistant endospores under appropriate conditions - Gas is a by-product of bacterial growth. Pathology: >The skin may be colonised around the buttocks and thighs with C. perfringens >If the organism gets into damaged tissue that is anaerobic, endospores will germinate to produce the vegetative bacteria that produce the toxins >These toxins cause cell damage and the products of the damaged tissue have systemic effects |
|
|
|
What is the treatment for gas gangrene?
|
>Surgery – immediate and must remove all affected tissue
- Amputation may be necessary >Benzylpenicillin – adjunct therapy and is not an alternative to surgery |
|
|
|
What is the clinical presentation of cellulitis and necrotising fasciitis?
|
>Cellulitis is a severe and rapidly spreading infection with a focus in the sub-dermal fat caused mainly by Streptococcus pyogenes (Group A strep), or a mixed infection of anaerobes
>Necrotising fasciitis is caused by the same organisms in the soft tissue below the dermis - There is an acute inflammatory response, much pain and erythema, which spread rapidly - The patient is 'toxic' due to bacterial enzymes and toxins that destroy tissue |
|
|
|
What is the treatment for cellulitis and necrotising fasciitis?
|
>Streptococcal infection – benzylpenicillin
>Necrotising fasciitis – radical excision of necrotic tissue, local and systemic antibiotics e.g. clindamycin + benzylpenicillin |
|
|
|
Define metaplasia.
|
>A reversible change in which one mature cell type is replaced by another
>Adaptive substitution >Reprogramming of stem cells e.g. Barrett's oesophagus |
|
|
|
Define dysplasia.
|
>Disordered growth
>Loss of uniformity in a population of cells and disorder of their normal architecture >Low grade changes may be reversible >High grade dysplasia is premalignant |
|
|
|
Define neoplasia.
|
>An acquired abnormality in which there is abnormal, uncoordinated and excessive cell growth which:
- persists after the initiating stimulus has been removed (autonomy) - which involves genetic alterations in the neoplastic cells |
|
|
|
What are the classifying differences in benign and malignant tumours?
|
BENIGN:
>Non-invasive >Remain localised >Slow growth rate >Closely resemble parent tissue histologically MALIGNANT: >Invasive >Spread directly and metastasise >Relatively rapid growth rate >Variable degree of resemblance to parent tissue |
|
|
|
Name five benign tumours of connective tissue.
|
1. Lipoma
2. Chondroma 3. Osteoma 4. Leiomyoma 5. Rhabdomyoma 6. Angioma |
|
|
|
Name five malignant tumours of connective tissue.
|
1. Liposarcoma
2. Chondrosarcoma 3. Osteosarcoma 4. Leiomyosarcoma 5. Rhabdomyosarcoma 6. Angiosarcoma |
|
|
|
How do carcinomas spread?
|
>Direct invasion of tissues
>Lymphatics to lymph nodes >Blood stream to bone and other organs, including lungs and liver |
|
|
|
How do sarcomas typically spread?
|
>Sarcomas spread by:
- direct invasion of tissues - from the blood stream to the lungs |
|
|
|
What is the commonest primary malignant tumour of bone?
|
>Osteosarcoma
>Only 100-120 cases in UK per year |
|
|
|
What is the commonest malignancy in bone?
|
>SECONDARY carcinoma
|
|
|
|
What are common sites of primary tumours?
|
1. Lung
2. Breast 3. Prostate 4. Kidney 5. Thyroid |
|
|
|
What is meant by differentiation (oncologically)?
|
>The extent to which neoplastic cells resemble comparable normal cells
>Well differentiated cells resemble the tissue of origin >Anaplasia = lack of differentiation |
|
|
|
What is the role of histopathology?
|
>It is predictive
- forms the basis of evidence based pathology >Gold standard for diagnosis - however not required if clinical diagnosis is feasible |
|
|
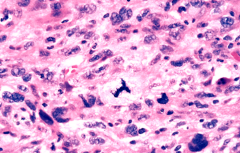
What is characteristic about these cells?
|
>Note the:
- nuclear pleomorphism - increased nuclear:cytoplasmic ratio - variation in nuclear shape and size - hyperchromasia - abnormal mitoses |
MALIGNANT TUMOUR NUCLEAR
|
|
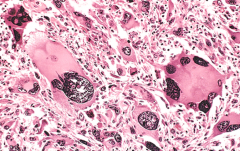
Identify this histopathology.
|
>A giant cell osteosarcoma showing:
- nuclear enlargement - multinucleation - anisonucleosis - prominent nucleoli |
OSTEOSARCOMA
|
|
|
Describe the pathogenesis of cancer.
|
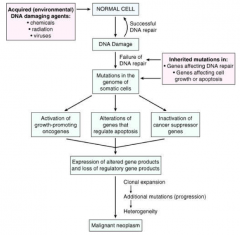
|
CANCER PATHOGENESIS
|
|
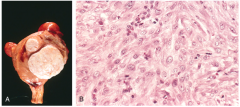
Identify this histopathology.
|
>World's commonest tumour
- a uterine fibroid (leiomyosarcoma) >Cells are recognisably smooth muscle cells with fairly uniform nuclei |
UTERINE FIBROIDS - LEIOMYOMA
|
|
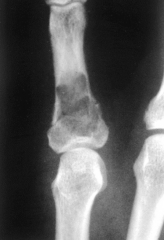
What is this pathology?
|
>A giant cell tumour of bone
|
GIANT CELL TUMOUR
|
|
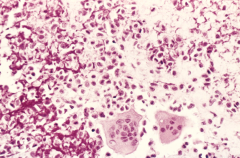
What is this histopathology?
|
>Giant cell tumour of bone
>Benign tumour - although giant cells are multinucleated the nuclei are regular and nucleoli aren't a feature |
GIANT CELL TUMOUR II
|
|

Identify this pathology.
|
>A chondrosarcoma arising at the end of a long bone (left) invading through the periosteum (right)
|
CHONDROSARCOMA
|
|
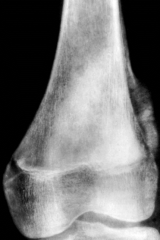
Identify this pathology.
|
>A classic radiograph of an osteosarcoma, showing the extraosseus 'hair on end' (or 'sun in splendour') and Codman's triangle
|
OSTEOSARCOMA X RAY
|
|
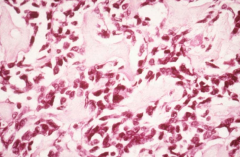
Identify this histopathology.
|
>Osteosarcoma
- Pink osteoid material between the bone is pathognomonic of osteosarcoma |
OSTEOSARCOMA CELLS
|
|
|
What is the difference between intramembranous and endochondral ossification?
|
>Intramembranous ossification replaces embryonic mesenchyme, endochondral ossification replaces cartilage
>Intramembranous ossification is largely an intrauterine process, endochondral continues up to adulthood |
|
|
|
What might be considered abnormal bone growth?
|
>Too little
>Too much >Problems with ossification |
|
|
|
What is achondroplasia?
|
>The most common disease of the growth plate and the major cause of dwarfism.
>Inheritance is autosomal dominant >80% of cases are new mutations of the FGF-3 gene >Most descriptions describe heterozygotes since homozygous infants normally die at birth or soon after from respiratory insufficiency due to a small chest cavity |
|
|
|
What are the morphological features of achondroplasia?
|
>Shortened proximal extremities and a trunk of relatively normal length
>A large head, prominent forehead and a depressed nasal root >Longevity, intelligence and reproductive status all normal |
|
|
|
What are the histological features of achondroplasia?
|
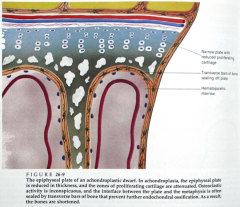
>Abnormal growth plates: the zones of chondrocyte proliferation and hypertrophy are narrowed and disorganised, with clusters of large chondrocytes rather than columns
>At the base of the growth plate, there is premature deposition of bone that seals the plate and prevents further growth >Intramembranous bone formation is not disrupted |
ACHONDROPLASTIC GROWTH PLATE
|
|
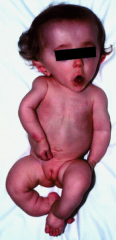
Identify this pathology.
|
>A group of phenotypically related disorders
>All caused by defects in type I collagen synthesis: mutations in genes that code for the alpha-1 and alpha-2 chains >Mutations are usually point substitutions and the more common ones are autosomal dominant - more severe lethal forms are autosomal recessive >Although bone formation is the most obvious defect ('brittle bone disease'), joints, eyes, skin and teeth are affected as well" |
OSTEOGENESIS IMPERFECTA
|
|
|
What is the morphology of osteogenesis imperfecta?
|
>There is a spectrum of severity
>Type II is uniformly fatal in utero or perinatally with multiple fractures >Type I, autosomal dominant, permits a normal lifespan but with increased fractures in childhood, blue sclerae, hearing defects, and small misshapen teeth >The underlying pathology in all cases is too little bone |
|
|
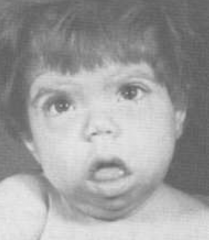
Identify this pathology.
|
Cretinism:
>Results from maternal iodine deficiency >Disproportionally short limbs >Large head due to delayed closure of fontanelles >Mental impairment |
|
|

What is Seckel syndrome?
|
>Bird headed or primordial dwarfism
>Microcephaly, receding forehead, prominent nose, downslanting palpebral fissures >Moderate - severe mental deficiency >Can survive to old age >Autosomal recessive >Mutation in the gene encoding ataxia-telangiectasia-related protein (ATR) which maps to chromosome 3a22.1q-24 |
PRIMORDIAL DWARFISM
|
|
What is this pathology?
|
>Growth hormone producing (somatotrope) adenoma of the pituitary occurring before epiphyseal fusion
>Excess GH after epiphyseal fusion leads to acromegaly |
GIGANTISM
|
|
|
What are some examples of currently used biological therapies for rheumatoid arthritis?
|
>TNFα inhibitors e.g. infliximab, etanercept (soluble TNFα receptor) and adalimumab
>IL-1 inhibitor e.g. anakinra (IL-1RA receptor antagonist) >IL-6 inhibitor e.g. tocilizumab (humanised anti-IL6R mAb >B cell depletor e.g. rituximab (anti CD20 chimeric mAb) >Inhibition of costimulation e.g. abatacept (binds to CTLA4-1g, which competitively binds with CD80 and CD86) |
|
|
|
What is polymyositis and what are the treatment options?
|
DISEASE:
>Fairly rare (prevalence 1 in 20,000) >Muscle disease associated with inflammation and autoantibodies to histidyl-tRNA synthetase >Associated skin involvement occurs in 50-60% of cases (dermatomyositis) TREATMENT OPTIONS: >Corticosteroids >Azathioprine >Methotrexate >Intravenous immunoglobulin |
|
|
|
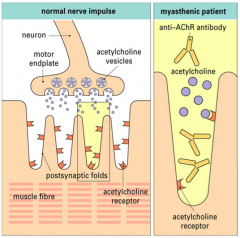
What is myasthenia gravis?
|
>Disorder of neuromuscular transmission characterised by abnormal fatigability of skeletal muscle ranging from transient double vision to life threatening respiratory paralysis
- prevalence is 1 in 10,000 - all ages can be affected - often presents with weakness in muscles controlling eye and eyelid movement - transient neonatal myasthenia in ~10& of babies born to myasthenic mothers due to transfer of maternal IgG antibodies NB. C.f. Graves disease NOT Hashimoto's thyroiditis (IgG sufficient to cause pathology) |
|
|
|
What is the pathogenesis of myasthenia gravis?
|
>Autoantibodies
>Blocking and or removal of the acetylcholine receptor" |
|
|
|
What are the treatment options in MG?
|
>Acetylcholinesterase inhibitors
>Corticosteroids >Azathioprine >Cyclosporine >Cyclophosphamide >Mycophenolate mofetil >Tacrolimus >Intravenous immunoglobulin >Plasma exchange >Thymectomy |
|
|
|
Which cells of the immune system are most important in autoimmune disease?
|
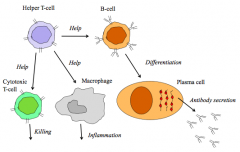
>Lymphocytes
- highly antigen specific - undergo tolerance, breakdown of which causes autoimmunity |
BREAKDOWN OF TOLERANCE
|
|
|
What is immunological tolerance and how does breakdown occur?
|
TOLERANCE (not 100% effective)
>Property of lymphocytes - both T and B cells can become tolerised >Central (bone marrow and thymus) and peripheral tolerance - clonal deletion (apoptosis) or clonal anergy (cell functionally inactive) >Suppression (regulatory/suppressor T cells) BREAKDOWN OF TOLERANCE: >Microbial / self antigen cross-reaction (molecular mimicry) >Pathogen alters structure or presentation of self antigen >Regulatory T cell failure >Cytokine dysregulation >Thymic defect |
MYASTHENIA GRAVIS
|
|
|
How does an RA joint differ from a normal joint?
|
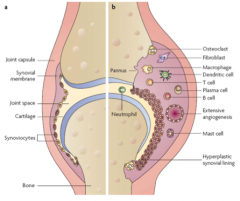
>Inflammation of synovium, vasculitis, oedema, inflitration by lymphocytes and macrophages, synovial proliferation and cartilage erosion.
|
RA JOINT
|
|
|
Describe the immunological elements of RA pathogenesis.
|
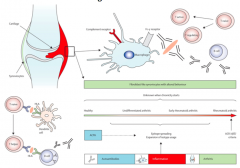
>Immune complexes
>Autoantigens: citrullinated peptides/proteins e.g. fibrinogen (post translational modification of arginine to citrulline) and IgG itself act as autoantigens, the latter leading to the production of 'rheumatoid factor' - IgG is a glycoprotein, may be recognised as antigen - the Fc part - NB. Rheumatoid factor is not diagnostic of RA |
RA PATHOGENESIS
|
|
|
What are common targets of currently used biological therapies for RA?
|
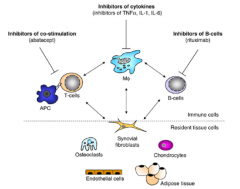
|
RA TARGETS
|
|
|
What occurs in Paget's disease?
|
>Characterised by very large (ie. Many nuclei), overactive OC
- cause may be infection with a paramyxovirus, similar to canine distemper virus or measles >Result is greatly accelerated bone resorption at certain focal sites, bone formation by OB is increased in an attempt to rectify the problem, but the new bone formed is disorganised >Paget's disease leads to painful deformities which are frequently hot to the touch >Can be treated quite effectively with calcitonin or bisphosphonates >Distribution of the disease is bizarre: almost unknown in many countries but is most common in the NW of England, where its occurrence correlates to some extent with dog ownership - recent evidence suggest association with mutations in cellular protein degradation apparatus |
|
|
|
What are the risk factors for osteoporosis?
|
1. menopause / oophorectomy (i.e. oestrogen deficiency)
2. hypothalamic amenorrhea (e.g. due to excessive exercise) 3. Hypogonadism in males 4. Genetic factors (e.g. white European) 5. Positive family history 6. Small build / low body weight 7. Sedentary lifestyle (moderate amounts of weight bearing exercise are beneficial) 8. Smoking, alcohol abuse (e.g. >25-30 units/week), excessive caffeine 9. Drugs (e.g. corticosteroids, heparin) 10. Excessive protein intake / chronic low level acidosis 11. AIDS, diabetes, respiratory disease, kidney disease, transplants |
|
|
|
What are the clinical features of cartilage wear?
|
>Most cartilage wear asymptomatic:
- absence of hyaline cartilage causes articular surfaces to alter their shape in response to load in a way that takes them away from normal - progressive change >Major cartilage loss? - disturbs mechanics of the joint by producing functional ligamentous laxity - especially in hinge joints - with bony outgrowth this may cause frictional synovitis - loss of articular cartilage leads to bone on bone articulation - resulting symptoms are pain, swelling, stiffness and in the long term instability - grinding bony crepitus >Mild to moderate? - no specific signs >Radiography? - loss of joint space - underlying bone shows sclerosis and systs - osteophytes may form at the joint margin and tend to be more localised than those which develop with advancing age alone - once cartilage loss is visible on a plain radiograph it usually progresses to total loss over months or years (1-2 for hip, 1-10 for knee) - in weightbearing joints this may be followed by osteonecrosis and collapse |
|
|
|
How is cartilage loss treated?
|
>Conservative care of joints with cartilage loss and associated new bone formation includes:
- explanation - analgesia for associated pain from soft tissue irritation - routine of regular exercise and sticks or walking aids - marked cartilage loss in the knee, with instability may benefit from a brace |
|
|
|
Is there a role for NSAIDS in cartilage loss?
|
>Maybe
>Presumably because some of it is mediated by prostaglandins - patient satisfaction response to NSAIDs is as good as in rheumatoid or similar arthropathies - toxicity of NSAIDs must however be borne in mind |
|
|
|
What is the definitive treatment of severe cartilage loss with night pain and immobility?
|
>Total joint replacement
- surgical treatment aimed specifically at osteophyte formation is most useful in the spine, when nerve root compression occurs - occasionally osteophyte removal is useful at peripheral joints |
|
|
|
A disease affecting more than half of women by the end of their lifes, with a clinical endpoint of fracture, usually of wrist, spine or hip is?
|
>Osteoporosis
|
|
|
|
What is the underlying cause of osteoporosis?
|
>Results from an imbalance between bone formation (by osteoblasts) and resorption (by osteoclasts), which leads to net loss of bone
>High turnover rate of trabecular bone makes it particularly sensitive, to such remodelling imbalances - end result is disruption and loss of trabecular microarchitecture - osteoporosis mainly affects skeletal sites with a high content of trabecular bone such as the vertebral bodies or the femoral neck |
|
|
|
"
In which sex are vertebral crush fractures more common? |
">Women x10 more common than in men
- disfiguring and painful - associated with 5 year mortality of 20-30% |
|
|
|
"
What is the prevalence and associated mortlity of NOF fractures? |
">20% mortality in first year
>Commonly occur in patients 70+ and show a 2:1 prevalence in favour of females |
|
|
|
In which races is osteoporosis less common?
|
>African or Caribbean - other ethnic groups have lower bone mass / density
|
|
|
|
What is the major feature of osteoporosis?
|
>Reduction in bone density
- detected by dual energy x-ray absorptiometry |
|
|
|
What is the major cause of bone loss in women?
|
>Oestrogen deficiency
- HRT primary treatment for postmenopausal osteoporosis - many additional benefits including the reduction of CV disease, reduction of hot flushes, improved libido, improved cognitive function, possible slowing of the development of some forms of senile dementia and possible reduction in large bowel cancer |
|
|
|
What are the possible risks of HRT?
|
>Small possible increased risk of breast cancer and with oral therapy, thrombosis
|
|
|
|
What treatments are available for osteoporosis?
|
>HRT
>Bisphosphonates >Selective oestradiol receptor modulators >Strontium ranelate >Parathyroid hormone >Calcitonin >Anti RANKL (denosumab) >Cathepsin K inhibitors >Antibodies to sclerostin and dickkopf-1 |
|
|
|
What is the mechanism of bisphosphonates?
|
>Bisphosphonates' mechanisms of action all stem from their structures' similarity to pyrophosphate
- A bisphosphonate group mimics pyrophosphate's structure, thereby inhibiting activation of enzymes that utilize pyrophosphate >Bisphosphonate-based drugs' specificity comes from the two phosphonate groups (and possibly a hydroxyl at R1) that work together to coordinate calcium ions - Bisphosphonate molecules preferentially "stick" to calcium and bind to it - largest store of calcium in the human body is in bones, so bisphosphonates accumulate to a high concentration only in bones >Bisphosphonates, when attached to bone tissue, are ""ingested"" by osteoclasts, the bone cells that break down bone tissue |
|
|
|
What are the adverse effects of bisphosphonates?
|
Associated with an increased risk of:
- AF - osteonecrosis of the jaw - inflamm. eye disease - possible oesophageal cancer - may result in fragility fractures of the femoral shaft |
|
|
|
What are SERMs?
|
>Selective oestradiol receptor modulators
- act like weak oestrogens - reduce risk of vertebral but not hip fractures - increase risk of venous thrombosis |
|
|
|
How does PTH benefit osteoporosis patients?
|
>Only agent that directly stimulates osteoblastic bone formation and thereby reduces fracture risk
- intermittent use only (prolonged release enhances resorption over new bone formation) - given by daily injection >VERY EXPENSIVE |
|
|
|
How does calcitonin benefit osteoporosis sufferers?
|
>Weak antiresorptive agent, intranasal
>Free from side effects but expensive |
|
|
|
What are anti RANKL antibodies?
|
">e.g. denosumab
- 6 monthly subcut injection - highly effective in osteoporotic fracture reduction - cell signalling systems common to different tissues :. Side effects are common - increase in infections, muscle pain and increased cholesterol |
|
|
|
What is cathepsin K?
|
>An osteoclast enzyme - inhibition of which (by odacatanib) prevents bone resorption
|
|
|
|
Name one osteoporosis therapy which targets the LRP/Wnt cell signalling system.
|
>Antibodies to either:
- sclerostin - dickkopf-1 |
|

