![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
Ways to make Alkanes in 1 Step |
1. Alkene + H2 in Pd, SYN 2. Alkyne + H2 in Pd, SYN 3. Thioether + H2 in Raney Nickel 4. Alkyl Halide + Grignard
|
|
|
Ways to make Alkenes in 1 Step |
1. E2 Elimination 2. E1 Elimination 3. Alkyne + H2 in Lindlar's Catalyst, Z 4. Alkyne + Li, NH3(l) in ROH, E |
|
|
Making alcohols |
1. Ketone + a) Grignard, b) H+ 2. Ketone + a) LiAlH4, b) H+ 3. Alkene + HX in H20, Markovnikov 4. Alkene + H3O+ 5. Alkene + a) BH3, b) HOO- ANTI-Markovnikov 6. Alkene + a) Hg(OAc)2, H20, b) NaBH4, NO REARRANGEMENT, MIX 7. Epoxide + H+, weak nucleophile 8. Epoxide + a) Strong Nuc, b) H+ 9. Alkene + a) OsO4, b) NaOH (Double Syn Alcohol) |
|
|
Making Ketones |
1. Primary alcohol + PCC 2. Secondary alcohol + PCC 3. Secondary alcohol + H2CrO4, H+, H20 4. Alkyne + HX, H20, Markovnikov 5. Alkyne + H+, H20, Markovnikov 6. Alkyne + HgSO4, H2SO4, H20, Markovnikov 7. Alkene + a) O3, b) DMS (makes two) 8. Alkyne + a) HB(Sia)2, b) HOO-, ANTI-Markovnikov |
|
|
Making a Carboxyllic Acid |
1. Primary alcohol + H2CrO4, H+, H20 2. Ester + H2O, H+ |
|
|
Cyclopropanation Epoxidation |
1. Alkene + carbene, SYN 2. Alkene + RCO3H (like mCPBA), SYN |
|
|
Making an Ester |
1. Carboxyllic Acid + Alcohol, H+ |
|
|
Silyl Ether Tosylate |
1. Alcohol + R3SiCl 2. Alcohol + TsCl |
|
|
Making Alkyl Halides |
1. Ether + HCl, HBr, HI 2. Alkene + HX 3. Alcohol + HX (Sn1) 4. Epoxide opening 5. Alcohol + PBr3, PCl3, PI3 6. Alkyne + HX (double) 7. Alcohol + SOCl2 8. Alkene + X2, Anti 9. Alkene + HX in ROOR, ANTI-Markovnikov |
|
|
Making thioethers |
1. RSHR + a) strong base, b) alkyl halide |
|
|
Epoxide Opening |
1. epoxide + a) strong base, b) H+ 2. epoxide + H+, weak base |
|
|
Additions to Alkenes: Which ones are Syn, Anti, Stereoisomerism? |
1. When electrophile does NOT have electrons, mix of stereoisomers 2. When electrophile has electrons, just ANTI 3. When electrophile and nucleophile are connected, just SYN 4. When one atom is electrophile and nucleophile, get SYN ring |
|
|
Reaction of alkene + a) BH3, b) HOO- |

|
|
|
Cyclopropanation Reaction |
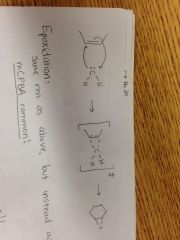
|
|
|
Alkyne + H+, H20 Reaction |
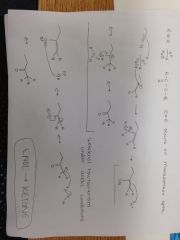
|
|
|
Alkyne + HgSO4, H2SO4, H20 |
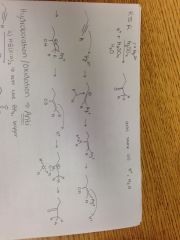
|
|
|
Alkyne + a) HB(Sia)2, b) HOO- Reaction |
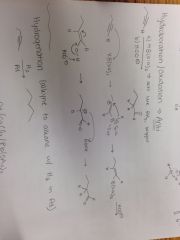
|
|
|
Fischer Esterification |

|
|
|
Ester Hydrolysis |

|
|
|
PCC & Primary Alcohol |
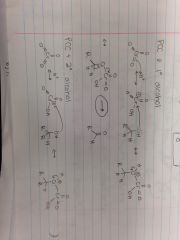
|
|
|
PCC & Secondary Alcohol |
|
|
|
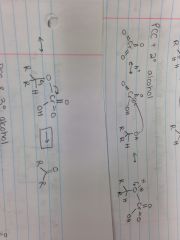
PCC & Tertiary Alcohol |

|
|
|
Chromic Acid + Primary Alcohol |

|
|
|
Mineral Acid Cleavage |

|
|
|
Acid Catalyzed Epoxide Opening |
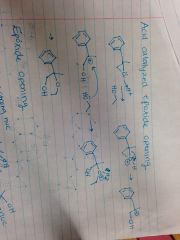
|
|
|
Strong Nucleophile Epoxide Opening |

|

