![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
|
What are the relative charges for Proton, Electron & Neutron? |
P: +1 E: -1 N: 0 |
|
|
What are the relative masses for Proton, Electron & Neutron? |
P: 1 E: 1/1840 N: 1 |
|
|
Where is the electron found in? |
Electron shells (outside nucleus) |
|
|
Where is the neutron found in? |
Inside the nucleus |
|
|
Where is the proton found in? |
Inside the nucleus |
|

Front (Term) |
2,8,8,8 |
|
|
The outermost electron shell is known as the.. |
valence shell |
|
|
The electrons in the valence electrons are known as.. |
valence electrons |
|
|
What is a molecule? |
Molecule is a group of 2 or more atoms chemically combined together |
|
|
The chemical formula of a molecule gives the ——— and ——— of atoms present |
type & number |
|
|
What is a compound & a mixture? |
A compound is 2 or more atoms chemically combined and a mixture consists of 2 or more substances (atoms, molecules, compounds) not chemically combined |
|
|
When a metallic atoms ——— electrons to form a fully filled valence shell, a ——— ion is formed |
loses, positive |
|
|
When a non-metallic atoms ——— electrons to form a fully filled valence shell, a ——— ion is formed |
gain, negatively |
|
|
What is a positive ion called? |
cation |
|
|
What is a negative ion called |
anion |
|
|
Atoms lose or gain electrons to achieve.. |
stable noble gas electronic configuration |
|
|
what is stable noble gas electronic configuration? |
fully filled valence |
|
|
Why are atoms electrically neutral? |
Atoms are electrically neutral because the have the same number of protons and neutrons |
|
|
What is a charged particle? |
Ions (The valence shell is not fully filled) |
|
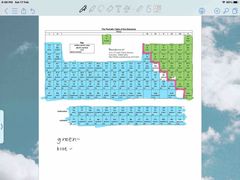
Front (Term) |
green - non metals blue - metals |
|
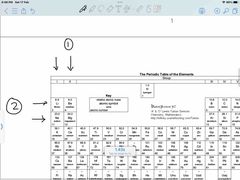
Front (Term) |
1- period 2- group |
|
|
When an ion gains electrons it should have a ——— symbol |
different |

