![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
183 Cards in this Set
- Front
- Back
|
what are the four types of diarrhea? for each one, is it common or uncommon in dogs and cats?
|
1. osmotic - common
2. secretory - uncommon 3. hypomotile (ileus) - uncommon 4. exudative - common |
|
|
what causes osmotic diarrhea? After how much time does it usually resolve?
|
unabsorbed nutrients (solutes) in the bowel cause passive diffusion of water into the gut lumen, thus creating a large volume of fluid or liquid stool (+/- statorrhea). Usually resolves after 24-36 hours.
|
|
|
what is exudative diarrhea? Causes? What is present in the exudate?
|
Altered mucosal permeability. The gut permeability barrier is lost, resulting of exudative loss of fluids into the large or small bowel. This can be caused by things leading to inflammation (e.g. GI erosions, ulcers). Exudate can contain fresh or melenic blood, serum proteins, and inflammatory cells.
|
|
|
what is protein losing enteropathy?
|
loss of serum protein as a result of exudative diarrhea
|
|
|
how does ileus cause GI problems?
|
- rapid intestinal transit (no segmentation/retentive peristalsis) leads to diarrhea
- small intestinal bowel overgrowth (SIBO) is a common sequela |
|
|
what is secretory diarrhea?
|
secretagogues (such as GI hormones, bacterial endotoxins, drugs, hydroxylated fatty acids, and unconjugated bile acids) cause fluid production by crypt epithelial cells, resulting in large volumes of diarrhea.
|
|
|
what is tenesmus?
|
a distressing but ineffective urge to evaculate the rectum or urinary bladder ("straining")
|
|
|
comment on how the following clinical signs relate to small bowel versus large bowel diarrhea:
- weight loss (chronic diarrhea) - frequency of defecation - volume per defecation - tenesmus - blood - mucus |
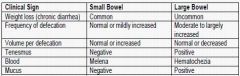
|
|
|
what are four general procedures used in the clinical management of diarrhea?
|
1. intestinal rest for 24-48 hours and maintain hydration status
2. IV, SQ fluids 3. offer small amounts of water, ice cubes, or electrolyte solutions (e.g. Rebound, Pedialyte) 4. small amounts of food 4-6 times per day |
|
|
what are five aspects of appropriate nutritional content of a diet formulated to manage diarrhea?
|
1. restricted fat
2. increased electrolytes 3. low fiber 4. highly digestible CHO (e.g. rice, corn) 5. normal amounts of protein |
|
|
what are the three basic types of commercial formulations available to treat diarrhea?
|
1. highly digestible, low fiber
2. hypoallergenic 3. fiber enhanced (binds water, SFCA production for flora and motility normalization) - NOTE: a combination of the three might be most effective |
|
|
term used to describe the effect of intestinal diseases that alter structure / function of mucosa plus lymphatics
|
Malabsorption
|
|
|
Failure of nutrients to pass across the intestinal wall
|
Malassimilation
|
|
|
Defects in intraluminal digestion as a result of gastric, pancreatic, or biliary dysfunction
|
Maldigestion
|
|
|
what is malabsorption?
|
Diseases that alter structure / function of mucosa plus lymphatics
|
|
|
what is malassimilation?
|
Failure of nutrients to pass across the intestinal wall
|
|
|
what is maldigestion?
|
Defects in intraluminal digestion as a result of gastric, pancreatic, or biliary dysfunction
|
|
|
Dilation and dysfunction of intestinal lymphatics
|
lymphangiectasia
|
|
|
what is lymphangiectasia and what are the two types?
|
- dilation and dysfunction of intestinal lymphatics
1. primary LGE - limited to intestine +/- chylothorax 2. secondary LGE - severe intestinal infiltrative disease (e.g. IBD, neoplasia, enteritis, obstruction of lymphatics) |
|
|
what is the pathogenesis of disease caused by lymphangiectasia?
|
- inability to absorb fat and release of fat from central lactules causes saponification of free fatty acids with calcium salts
- leakage of water and albumin from lymphatics - malabsorption of vitamins A, D, E, K - loss of lymphocytes, cholesterol, and fat |
|
|
what are the clinical signs of lymphangiectasia?
|
- weight loss and poor BCS (pitting edema, ascites, pleural effusion from protein loss) - note protein loss is in part due to rupture of intestinal lymphatics
- chronic diarrhea, steatorrhea, increased stool production - pan hypoproteinemia, hypocholesterolemia, lymphopenia, hypocalcemia - intestinal biopsy: dilated, chyle-engorged lacteals and sub-mucosal lymphatics, edema, lipogranulomas |
|
|
which dog breeds are predisposed to lymphangiectasia?
|
Yorkies, Maltese, Dachshund
|
|
|
what are the four primary dietary management goals to treat lymphangiectasia?
|
1. minimize lymph flow
2. LIMIT FAT INTAKE 3. reduce lacteal and lymphatic distention 4. minimize protein loss |
|
|
what are seven key nutrient factors for management of lymphangiectasia?
|
1. LOW FAT
2. medium chain trig supplementation (improves BCS) 3. high levels of high-quality protein (may be hydrolyzed or supplemented, such as cooked egg whites) 4. avoid insoluble fiber because it interferes with intraluminal fat digestion 5. high digestibility and low residue 6. IM vitamins A, D, E q 3 months 7. consider IV calcium (also Mg, Zn, Cu) |
|
|
what are some clinical signs of pancreatic exocrine insufficiency (PEI/EPI)
|
- poorly digested food
- chronic small bowel diarrhea - frequent defecation of foul smelling, greasy, pale, voluminous stools - weight loss / failure to thrive - polyphagia, pica, coprophagia - hemorrhages (vitamin K deficiency) |
|
|
what breeds of dogs and cats are predisposed to PEI?
|
- German shepherd dog, rough-coated collie
- Siamese cat |
|
|
what are the two types of PEI (exocrine pancreatic insufficiency) and their pathogenesis?
|
1. Juvenile PEI - atrophy of pancreatic acinar tissue; clinical signs develop at 6-18 months
2. Acquired PEI - inflammation and fibrosis associated with end-stage pancreatitis. +/- diabetes mellitus |
|
|
how does exocrine pancreatic insufficiency (PEI/EPI) cause disease?
|
- failure of intraluminal digestion and ineffective nutrient utilization
- impaired mucosal enzyme activity (lack of tropic pancreatic secretions; osmotic or secretory diarrhea) - secondary concerns such as small intestinal bacterial overgrowth |
|
|
what are six key nutritional factors when treating an animal for PEI (exocrine pancreatic insufficiency)?
|
1. highly digestible CHO and protein
2. restricted fat 3. consider supplementing MCT into diet 4. low fiber diet 5. parenteral fat soluble vitamins (ADEK) 6. B12 injections |
|
|
why does PEI cause diarrhea?
|
because of the high osmotic load: osmotic diarrhea
|
|
|
what is an effective additive for enteral diets to treat PEI? how is it used?
|
pancreatic enzymes, mixed with food immediately prior to feeding
|
|
|
for a patient with PEI, how much should be fed to them?
|
DER for optimal BW + 20%
|
|
|
why would you want to add antacids or histamine blockers to a diet containing pancreatic enzymes?
|
because gastric acids can break down the enzymes.
|
|
|
what are three synonyms for the disease in which the kidney progressively loses function over a period of time?
|
1. chronic kidney disease (CKD)
2. renal insufficiency 3. chronic renal failure |
|
|
what is the most common cause of death in elderly animals
|
chronic renal failure
|
|
|
what are the eight main functions of the kidney?
|
1. excrete nitrogenous waste (urea, creatinine)
2. maintain systemic blood pressure with the R-A-A system (renin secreted by the juxtaglomerular cells) 3. pH balance (only organ that secretes H+) 4. maintain fluid balance in the body 5. maintain electrolyte balance in the body 6. maintain calcium balance via Vitamin D metabolism 7. control of circulating red cell mass 8. detox of endogenous and exogenous substances |
|
|
what is the prevalance of renal insufficiency in dogs and cats?
|
- dogs - 10%
- cats - 30% |
|
|
what are seven clinical signs of chronic renal failure?
|
1. weight loss
2. PU/PD 3. poor haircoat 4. lethargy 5. inappetance 6. vomiting/diarrhea 7. nocturia |
|
|
why can renal insufficiency cause vomiting and/or diarrhea?
|
because urea is toxic to body cells, and the cells of the GI tract are especially sensitive to the effects of uremia
|
|
|
what are six blood chemistry indicators of renal insufficiency?
|
1. elevated BUN
2. elevated creatinine 3. hyperphosphatemia 4. hyper/hypokalemia 5. metabolic acidosis 6. hypoalbuminemia |
|
|
what are seven urinalysis indicators of renal insufficiency?
|
1. impaired concentrating ability (USG)
2. proteinuria 3. cylinduria (protein casts in the urine) 4. renal hematuria 5. abnormal pH 6. glucosuria 7. cystinurea (abnormal amount of cystine - aka cysteine disulfide dimer) |
|
|
comment on the prevalance and reversibility of acute versus chronic renal disease
|
- acute: less common; reversible
- chronic: most common; irreversible |
|
|
what fraction of nephrons must be lost before blood BUN or creatinine will increase?
|
75%
|
|
|
which three parts of the nephron are most affected by renal disease?
|
glomeruli, tubules, interstitium
|
|
|
what are two causes of glomerulonephropathy?
|
1. glomerulonephritis
2. amyloidosis |
|
|
what is the end result of chronic glomerulonephropathy? How is protein involved in renal failure caused by this pathology?
|
- glomerular sclerosis
- proteinuria --> hypoalbuminemia |
|
|
what is an abnormal urine protein to creatinine ratio? why do we use a ratio instead of absolute concentrations?
|
- >0.4 is considered abnormal
- we use a ratio because absolute concentration is meaningless given that urine can be concentrated or diluted |
|
|
what happens to intact glomeruli and GFR when other glomeruli start to die out?
|
- increase glomerular pressure
- GFR decreases because there are less of them |
|
|
what is the cascade of events leading to death as a result of chronic renal failure?
|

|
|
|
what are the stages of chronic renal failure and the level of azotemia?
|
Stage I: non-azotemic CRF
Stage II: mild renal azotemia Stage III: moderate renal azotemia Stage IV: severe renal azotemia |
|
|
how does protein intake affect the onset, prevention, and progression of renal disease?
|
- it does not in healthy animals
- it does not prevent renal disease - it is unlikely it slows the progression of renal disease unless there is proteinuria, and even that is not clear |
|
|
what are five goals of dietary management of patients in chronic renal failure?
|
1. prevent anorexia and weight loss
2. prevent secondary hyperparathyroidism 3. avoid protein malnutrition 4. limit the production of uremic toxins 5. maintain an adequate GFR |
|
|
what is rubber jaw?
|
in animals with chronic renal failure, demineralization of the mandible bone caused by secondary hyperparathyroidism
|
|
|
at what stages of renal failure in dogs and cats should nutritional therapy be started?
|
- dogs: Stages III-IV
- cats: Stages: II-IV |
|
|
what is the #1 most important nutrient in treating chronic renal failure?
|
water
|
|
|
why is it desirable to feed a high fat diet to patients in chronic renal failure? how much fat is in a typical renal diet?
|
- higher energy content (prevents protein catabolism and azotemia)
- highly palatable - meets DER in a smaller volume - minimizes gastric distention (vomiting) - typically 30-60% *energy* from fat |
|
|
how can protein restriction in patients with renal failure possibly slow the progression of the disease?
|
- delay of azotemia
- delay of uremia - delay of acidosis |
|
|
why can excessive protein restriction in patients in chronic renal failure make the disease worse?
|
because the body will start to catabolize lean muscle and further increase BUN and other nitrogenous waste products
|
|
|
what is the effect of reduced phosphorus intake in chronic renal failure?
|
- slows the progression of the dz in dogs
- prolongs survival rate in dogs - less severe renal lesions in cats |
|
|
how can chronic renal failure cause secondary hyperparathyroidism?
|
there is a decreased production of 1,25(OH)2-vitamin D in the kidney, and thus reduced dietary calcium absorption
|
|
|
how is renal disease made worse by
- sodium excess? - sodium deficit? |
- sodium excess leads to hypertension (increased stress on the nephrons)
- sodium deficit leads to lower water volume in the body --> lower GFR --> azotemia |
|
|
which nutrients are of primary concern in managing chronic renal failure?
|
protein, water, Ca, P, Na, K, Vitamin D, omega-3 and 6 FA, fiber, water soluble vitamins
|
|
|
in what species is hypokalemia prevalent with chronic renal faliure?
|
cats
|
|
|
in chronic renal failure, what three things contribute to metabolic acidosis?
|
1. decreased H+ excretion and reduced bicarb reabsorption
2. acid from sulfur containing amino acids (Met, Cys) 3. acid from catabolism of muscle protein |
|
|
dissolution of bone minerals due to chronic renal failure
|
renal osteodystrophy
|
|
|
when managing renal failure, if muscle catabolism cannot be resolved by managing protein intake, what else can be supplemented into the diet?
|
alkalinizing agents:
- Na bicarb - Ca carbonate - Potassium citrate (not for hyperkalemic patients) |
|
|
with regards to omega fatty acids, what aspect of them is important with regards to managing chronic renal failure?
|
want a low n-6:n-3 ratio:
- < 5:1 in dogs - < 7:1 in cats - we want them in sufficient quantity |
|
|
what three effects does supplementation of n-3 fatty acids have on chronic renal failure?
|
1. reduces inflammation
2. lowers systemic blood pressure 3. preserves renal function by decreasing proteinuria |
|
|
how does adequate fermentable fiber intake help patients in chronic renal failure?
|
microbial fermentation will remove urea from the digesta, thus decreasing urea reabsorption
|
|
|
what are three clinical signs of acute renal failure?
|
1. insulin resistance (hyperglycemia)
2. acidosis 3. catabolism |
|
|
what are feeding strategies for managing acute renal failure initially and for recovery?
|
INITIALLY (first 24-48 hours)
1. fluids and electrolytes 2. determine feeding method: parenteral vs. enteral 3. avoid high levels of amino acids and glucose RECOVERY 1. maximize energy from fat and CHO 2. adjust protein intake based on degree of azotemia |
|
|
in patients with proteinuric renal failure, what are three aspects of dietary management?
|
- feed reduced-protein foods designed for patients with this condition
- restrict Na (hypertension) - n-3 fatty acids (proven to reduce proteinuria) |
|
|
which protein sources commonly cause allergic reactions in dogs? cats?
|
- dogs: beef, dairy, fish, wheat
- cats: beef, chicken, chicken egg, soy, corn |
|
|
what two properties of a protein molecule determines its ability to induce hypersensitivity?
|
1. size (MW)
2. structure (conformation) |
|
|
what are six normal substances in the GI tract that protect against antigens?
|
1. GI acids
2. mucous layer 3. epithelial cell barrier 4. digestive enzymes 5. bile salts 6. IgA |
|
|
what three GI disorders commonly lead to protein hypersensitivity?
|
1. maldigestion
2. inflammatory mucosal disorders (breakdown of epithelial junctions) 3. T-suppressor cell dysfunction |
|
|
what is the typical age for onset of food allergies?
|
- 4 months - 14 years old!
- < 6 months is most common |
|
|
what are six clinical signs of food allergy?
|
1. non-seasonal pruritic dermatitis
2. otitis externa 3. intermittent vomiting 4. diarrhea 5. colitis 6. borborygmus (intestinal rumbling) |
|
|
what are the three major dietary strategies to manage food allergies?
|
1. novel protein / CHO (duck, rabbit, barley, oats, etc.)
2. modified protein strategy (hydrolyzed so it is low MW) 3. limited antigen exposure (i.e. allergy may be caused by additives, low digestibility, excess protein) |
|
|
how long does a single diet trial (for food allergies) last?
|
- 4-12 weeks for dermatological diseases
- 2-4 weeks for GI disease |
|
|
if a diet trial for food allergy is successful, what should you do next?
|
"challenge" with the original food/protein to see if clinical signs return. If they do, then it is most likely a food related disease
|
|
|
what are five common non-immunologically related causes of a food-related adverse reaction?
|
1. food poisoning (e.g. excess Vit A & D; spoilage; specific foods such as onion, chocolate, grapes; toxic preservatives)
2. reaction to additives (e.g. coloring) - controversial 3. vasoactive amines in food (e.g. spoiled fish, tomato, avocado, cheese, liver, sausage) 4. CHO intolerance (e.g. lactose) 5. dietary indiscretion (gluttony, pica, got into the trash) |
|
|
what are the three most common diseases of the heart (in small animals) and species/breed predisposition?
|
1. chronic valvular disease (CVD; endocardidits) - small to medium sized dogs
2. dilated cardiomyopathy & pericardial disease - large breed dogs (DOBIES!) 3. hypertrophic cardiomyopathy - cats |
|
|
what is syncope?
|
loss of consciousness ("fainting") due to inadequate blood flow to the brain - can be due to cardiac problems
|
|
|
what are NINE (yes, nine) clinical signs of cardiac disease?
|
1. coughing
2. dyspnea 3. inability to sleep comfortably 4. "seizures (fainting)" (syncope) 5. weight loss 6. abdominal distension 7. weakness 8. exercise intolerance 9. poor growth (congenital heart dz) |
|
|
inadequate cardiac output to meet the body's needs
|
cardiac failure
|
|
|
what are four clinical signs of congestive heart failure?
|
1. exercise intolerance
2. pulmonary and/or systemic venous congestion 3. low cardiac output 4. retention of sodium and water (body's attempt to compensate for low cardiac output) |
|
|
what two nutrient deficiencies have been linked to myocardial disease?
|
taurine and L-carnitine
|
|
|
how can renal disease lead to heart failure?
|
renal disease --> systemic hypertension (renin-angiotensin-aldosterone) --> ventricular hypertrophy --> heart failure
|
|
|
in what three ways does angiotensin II lead to high blood pressure?
|
1. stimulates aldosterone secretion --> sodium and water retention
2. vasoconstriction 3. stimulates thirst |
|
|
what stimulates cardiac cachexia? how can this be differentiated from simple starvation?
|
- malnutrition leads to muscle catabolism and weight loss
- simple starvation will be accompanied by mobilization of body fat to preserve lean body mass; cardiac cachexia will show no fat catabolism and continued loss of lean muscle mass |
|
|
what are some aspects of a good diet to manage cardiac cachexia?
|
- palatable
- adequate calories and protein - ω-3 fatty acids |
|
|
what is the "obesity paradox"?
|
humans with heart failure have higher survivability if they are slightly overweight
|
|
|
for feeding an obsese animal with heart failure, what two aspects of feeding are important?
|
1. feed for weight loss
2. low sodium |
|
|
at what BCS should you implement a weight loss program in a patient with heart failure?
|
>6/9
|
|
|
explain the various stages of heart failure?
|
1a - asymptomatic - signs of heart dz, but no signs of compensation visible in imaging
1b - asymptomatic - signs of dz and evidence of compensation can be seen in radiography/ECG 2. mild to moderate heart failure - symptomatic 3a. advanced heart failure - home care is possible 3b. advanced heart failure - hospitalization mandatory |
|
|
what are clinical signs of hypokalemia in heart failure? what can cause this? how is it treated?
|
- arrhythmia, muscle weakness, toxicity to digitalis administration (used to increase myocardial contraction)
- caused by anorexia, diuretics - Tx: oral potassium supplementation |
|
|
what can cause hyperkalemia in patients undergoing treatment for heart failure? how is this treated?
|
- ACE inhibitors, potassium-sparing diuretics
- withdraw any K+ supplements, consider other diuretics |
|
|
hypomagnesemia in patients with cardiac failure:
- clinical signs - causes - treatment |
- arrhythmia, low cardiac contractility
- caused by diuretics - change diet, supplement Mg into diet |
|
|
who is most susceptible to heart disease due to taurine deficiency?
|
cats
|
|
|
taurine in the cat:
- how does it regulate heart function? - clinical signs of taurine deficiency - sources of taurine in the diet |
- regulates Ca and K in the cardiac myocytes
- clinical signs: feline central retinal degeneration, platelet abnormalities, feline dilated cardiomyopathy - sources: meat, organ meat, feline diets |
|
|
what type of heart disease can be caused by taurine deficiency?
|
dilated cardiomyopathy
|
|
|
comment on dog breeds with respect to taurine and dilated cardiomyopathy
|
- positive correlation of taurine deficiency and DCM in cocker spaniels, golden retrievers, labs, St. Bernard, English setter
- taurine deficiency without DCM: Newfoundlands - dobies and boxers with DCM: taurine deficiency is an unlikely cause |
|
|
how does ω-3 FA supplementation help to manage heart disease?
|
- reduces cachexia
- improves food intake - reduces arrhythmogenesis - ω-3 supplementation BEFORE chronic heart failure is cardioprotective |
|
|
what four nutrients are required to synthesize L-carnitine?
|
1. lysine
2. methionine 3. Vitamin B6 (pyridoxyl phosphate) 4. Vitamin C |
|
|
in which dog breeds has L-carnitine deficiency been associated with dilated cardiomyopathy?
|
dobie, boxer, cocker spaniel
|
|
|
a deficiency of Coenzyme Q10 has been suggested as a possible cause of what disease?
|
dilated cardiomyopathy
|
|
|
what are the most common urolith types in dogs?
|
struvite and oxalate
|
|
|
what are the five most common types of canine uroliths?
|
1. struvite
2. oxalate 3. urate 4. cystine 5. mixed |
|
|
in dogs, how is breed a risk factor for urolith formation and what three mechanisms have been proposed to account for this?
|
- small breeds are at higher risk than large breeds
LARGE BREEDS HAVE: 1. larger urinary volume per unit BW^0.75 2. more urinations per day 3. lower urinary pH |
|
|
comment on gender predisposition to urolith formation
|
males > females (females have a larger and shorter urethra)
|
|
|
what are the two most important nutritional factors in urolith formation?
|
1. diet
2. water consumption |
|
|
what nutrient factors are associated with struvite urolith formation in dogs?
|
- high magnesium and high phosphorus
|
|
|
what nutrient factors are associated with calcium oxalate urolith formation in dogs?
|
- dry, ACIDIFYING diet
- high calcium - high oxalate - high vitamin C (oxalate is the metabolite of vitamin C) |
|
|
what nutrient factor has been associated with ammonium urate urolithiasis in dogs?
|
high purines in the diet (recall that awful pathway from biochem that involves uric acid formation... it is supposed to be converted to allantoin)
|
|
|
what drugs and systemic disorders can predispose a patient to urolithiasis due to increased calcium excretion?
|
DRUGS
1. urinary acidifiers 2. diuretics 3. glucocorticoids DISORDERS 1. hypercalcemia: hyperparathyroidism (dog); idiopathic hypercalcemia (cat) 2. Cushing's (hyperadrenocorticism) - elevated glucocorticoids |
|
|
what types of systemic disorders can cause ammonium urate uroliths?
|
- defects in purine metabolism
- portal vascular abnormalities - hepatic dysfunction |
|
|
what breed of dog is especially susceptible to ammonium urate uroliths?
|
dalmations
|
|
|
what can cause xanthine uroliths in dogs?
|
long-term treatment with allopurinol
(allopurinol is a xanthaine oxidase inhibitor used to treat gout in dogs) |
|
|
abnormal frequency of urination
|
pollakiuria
|
|
|
what is pollakiuria?
|
abnormal frequency of urination
|
|
|
what disorders of micturition can be indicative of uroliths?
|
- incontinence
- dysuria - pollakiuria - anuria - hematuria |
|
|
why can a UTI cause secondary urolithiasis?
|
it forms a nidus
|
|
|
how is urolithiasis diagnosed clincally?
|
- history
- palpation - radiography (ammonium urate is not radiodense), ultrasound - UA (sediment, pH, SG) - quantitative analysis of crystals |
|
|
which type of urolith cannot be detected by x-rays?
|
ammonium urate
|
|
|
what three conditions are required for urolith formation?
|
1. site of nucleation (nidus)
2. supersaturation/oversaturation 3. favorable pH and temperature |
|
|
what is a common cause of urine supersaturation?
|
overconcentrated urine due to urinary retention
|
|
|
a term used to describe abnormal concentrations of substances in the urine that may add to the size of a a urolith, but will not spontaneously form crystals.
|
metastable supersaturation
|
|
|
a term used to describe abnormal concentrations of substances in the urine that may spontaneously cause crystallization and/or rapid crystal growth
|
labile supersaturation
|
|
|
a term used to describe the precence of urolith-forming substances in the urine that are not in sufficient concentration to form uroliths
|
undersaturation
|
|
|
what are the normal components of struvite uroliths? gross appearance? urine pH?
|
- Mg, NH4, PO4, occasionally Ca
- white to yellow, soft or hard, smooth or rough - form in alkaline urine |
|
|
comment on the following risk factors for struvite urolith formation in dogs:
- gender - age - concurrent disease - breed |
- gender: female (85-95% prevalence)
- age: 1-8 years - concurrent disease: UTI in 2/3rd of cases ("sterile" versus "infection-induced" struvite) - breed: minitaure schnauzer, bichon, shih tzu, miniature poodle, lhasa apso - SMALL BREEDS |
|
|
why would a UTI contribute to struvite urolith formation
|
urease producing bacteria such as Staphylococcus and Proteus can alkalinize the urine
|
|
|
if struvite uroliths are present in the dog, what is the main goal of dietary management?
|
to dissolve the stone
|
|
|
what aspects of diet can be used to dissolve struvite uroliths?
|
- diuresis
- increased water intake - restrict Mg, PO4, protein - add sodium (increases thirst --> higher urine output) - acidify the urine |
|
|
in the dog, how long does it take to dissolve struvite
- uroliths? - nephroliths? |
- uroliths: 8-20 weeks (avg. 3.5 mos)
- nephroliths: 67- 300 days (avg. 6 mos) |
|
|
in the dog, how long should you keep feeding a calculolytic diet after negative radiographs/ultrasound?
|
1 month
|
|
|
what is a calculolytic food?
|
a diet used to dissolve uroliths, especially struvite
|
|
|
what is a major reason why a calculolytic diet will fail to prevent canine struvite urolithiasis?
|
because they have a concurrent UTI
|
|
|
what are four common types of uroliths caused by purine metabolism?
|
- ammonium urate
- Na urate - Ca Urate - xanthine |
|
|
what is the pH of urine that forms urate stones in the dog?
|
acidic
|
|
|
what are the four risk factors for urate crystal formation in the dog?
|
1. gender: male >85%
2. age <1 year with PSS; avg 3.5 years without PSS 3. concurrent disease: PSS,liver dz, +/- UTI 4. breed: dalmation, English bulldog, miniature schnauzer (PSS), yorkie (PSS) |
|
|
what two enzyme defects/deficiencies can lead to purine-type uroliths in the dog?
|
- xanthine oxidase
- uricase |
|
|
in the normal dog, what is the end-product of purine metabolism?
|
allantoin
|
|
|
what is the origin of the urate that is found in urate crystals?
|
purines from DNA
- damage to DNA (e.g. cancer) - diets high in DNA - methylpurines such as theophyllene (a veterinary drug) |
|
|
what places does the ammonium originate in ammonium urate uroliths?
|
- ammonia from PSS (protein breakdown)
- bacterial fermentation of urea - ammonium produced by the kidney to excrete H+ |
|
|
what are five basic ways to promote dissolution of ammonium urate crystals?
|
1. promote diuresis
2. restrict dietary purine 3. restrict dietary protein 4. control UTI 5. maintain a neutral-alkaline urine |
|
|
if you manage ammonium urate crystals with xanthine oxidase inhibitors, what else is necessary to provide and why?
|
- give allopurinol and a purine restricted diet
- minimizes risk of xanthine stone formation |
|
|
why do we want to avoid xanthine stones?
|
because they cannot be dissolved
|
|
|
how long does it take an ammonium urate stone to dissolve with proper dietary management?
|
4-40 weeks (mean 14.2 weeks)
|
|
|
in what four basic ways can ammonium urate uroliths be prevented clinically?
|
- PSS surgery (if extra-hepatic)
- low protein diet that promotes the formation of dilute alkaline urine - avoid high protein, acidifying diets - monitor daily urate excretion |
|
|
calcium oxalate uroliths risk factors in the dog
|
- gender: males > females
- age: 6-12 years - breed: miniature schnauzer, lhasa apso, shih tzu, yorkie, miniature poodle |
|
|
in which pH conditions do calcium oxalate uroliths grow?
|
acidic to neutral
|
|
|
what two main urine abnormalities lead to the formation of calcium oxalate uroliths?
|
1. hypercalciuria
2. hyperoxaluria |
|
|
what are three major pathophysiological processes that lead to hypercalciuria?
|
1. intestinal hyperabsorption (e.g. hypervitaminosis D)
2. renal leak (defect in calcium reabsorption from the renal tubule) 3. excessive skeletal mobilization of Ca (resorptive) |
|
|
what are four major causes of hyperoxaluria?
|
1. high dietary oxalates
2. high Vitamin C (oxalate is the metabolite of vitamin C) 3. glycine and serine 4. restriction of dietary calcium |
|
|
why would restriction of dietary calcium lead to calcium oxalate uroliths?
|
- because calcium will be mobilized from the bones and excreted in the urine
- lower intestinal calcium oxalate elimination leads to ↑oxalate absorption |
|
|
why can vitamin C intake lead to calcium oxalate uroliths?
|
because oxalate is the metabolite of vitamin C
|
|
|
why can calcium oxalate crystals be a big problem?
|
because they cannot be dissolved
|
|
|
by what two mechanisms can a crystallization inhibitor help to mitigate calcium oxalate urolith formation? what are three examples of crystallization inhibitors?
|
1. form soluble salts with Ca and Oxalic acid
2. Interfere with the binding of Ca and oxalic acid (e.g. chelating agents) - examples: Citrate, Mg, pyrophosphate |
|
|
what five nutrients are critical for preventing calcium oxalate formation in the dog? comment on what do do with each nutrient?
|
1. Moderate (adequate) dietary Calcium
2. Lower Oxalate 3. Avoid excess Vitamin C 4. Avoid Excess Vitamin D 5. Do not restrict phosphorus |
|
|
what are four nutrients/drugs/supplements to add to the diet in preventing calcium oxalate urolith formation
|
1. potassium citrate
2. thiazide diuretic (e.g. hydrochlorothiazide) 3. urolith inhibitors (avoid aciduria) 4. water - maintain dilute urine |
|
|
what are three common ways to dilute the urine?
|
1. diuretics
2. canned food (higher water content) 3. sodium chloride (increases thirst) |
|
|
what is cystine urolithiasis? risk factors
|
an inborn error in metabolism of certain breeds of dogs that deposits cystine crystals (cysteine disulfide) in the urine
- breed: English bulldog, dachshund, basset hound, newfoundland - age: 2-5 years - acidic urine |
|
|
what are four ways to manage cystine urolithiasis? length of treatment?
|
1. reduce dietary protein
2. diuresis 3. alkalinizing diet 4. thiol-containing drugs - this is lifelong dietary management |
|
|
what is FLUTD?
|
feline lower urinary tract disease
|
|
|
what are the three most common causes of feline lower urinary tract disease? comment on prevalence.
|
1. urolithiasis (25%)
2. urethral plugs (20%) 3. idiopathic (55%) |
|
|
what are seven non-idiopathic risk factors for FLUTD?
|
1. low urine pH
2. dietary mineral intake 3. dry food/decreased water intake 4. obesity 5. sedentary/indoor lifestyle 6. infection 7. neuroendocrine disorders |
|
|
why are obese cats more prone to FLUTD?
|
obese cats do not "clean their intimate areas" as well
|
|
|
what are seven clinical signs of FLUTD?
|
1. stranguria/dysuria
2. pollakiuria 3. periuria 4. hematuria/pyuria 5. anuria 6. urethral obstruction 7. +/- crystalluria |
|
|
what are five risk factors for FLUTD?
|
1. males > females
2. overweight females 3. age: 1-10 years 4. breed: burmese, persian, himalayan breeds - calcium oxalate 5. concurrent disease |
|
|
what are the two most common feline uroliths?
|
1. struvite
2. calcium oxalate |
|
|
what are five problems with the urinary tract that can lead to FLUTD?
|
1. infection & inflammation
2. neoplasia, anatomic abnormalities (e.g. hit by car) 3. crystals and/or uroliths 4. mucus (matrix) plugs 5. crystalline (matrix) urethral plugs |
|
|
what is required to form the matrix for mucus and crystalline urethral plugs?
|
infection, inflammation, interstitial cystitis
|
|
|
what is the main mineral in urethral plugs?
|
struvite
|
|
|
why does feeding multiple meals to a cat prevent urolith formation?
|
because it dampens the effect of postprandial alkaline tide
|
|
|
what causes postprandial alkaline tide?
|
gastric acid release increases blood bicarbonate, which is excreted into the kidney and alkalinizes the urine
|
|
|
how do you get rid of calcium oxalate crystals?
|
surgery
|
|
|
what are the three basic types of urolith-preventing diets?
|
1. struvite diets (acidifying)
2. calcium oxalate diets (alkalinizing) 3. diets that treat both |
|
|
what is the best preventative for feline idiopathic cystitis?
|
nobody knows
|
|
|
what should you feed a cat with idiopathic cystitis, and how long does the disease take to clear up?
|
- feed a canned food
- resolves in 7-10 days |
|
|
what are two pathogenic processes of calcium oxalate formation from overzealous feeding of acidifying diets?
|
1. acidemia: mobilizes Ca from bone --> hypercalcuria
2. vitamin B deficiency - hyperoxaluria |
|
|
what is the most important aspect of preventing recurrence of a compound urolith?
|
minimize the recurrence of minerals comprising the nucleus of the urolith
|

