![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
85 Cards in this Set
- Front
- Back
|
Define transition metal?
|
d electron shell are being filled therefore generally ignore Sc(III) and Zn(II) which are d⁰ and d¹⁰ |
|
|
What is the value of ∆t relative to ∆o?
|
∆t = 4/9 ∆o
|
|
|
Low spin vs high spin?
|
if ∆ is greater or less than the spin pairing energy |
|
|
Why is ∆ larger for metals in higher oxidation states?
|
The higher charge on the metal exerts more attraction to the ligands resulting in shorter bond lengths and hence more interaction between d-orbitals and ligand charge
|
|
|
Why does colour vary with ligand?
|
higher field ligands increase ∆ increasing homo-lumo gap decreasing wavelength |
|
|
Ligand Field Theory?
|
extension of crystal field theory using parameters from spectroscopic date rather than describing ligands as point negative charges |
|
|
Draw σ-bonds only diagram for metal ion and six
σ-orbitals? |
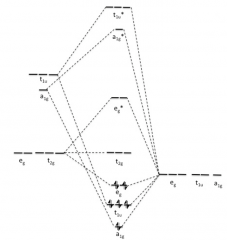
|
|
|
Draw σ- and π- bonds diagram for metal ion and six σ-orbitals and π-interactions
|
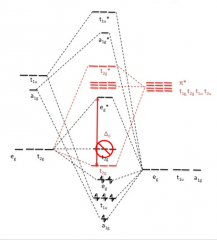
|
|
|
describe mulliken symbols?
|
-a is symmetric with respect to Cn, b is antisymmetric -1 and 2 mean symmetric and antisymmetric respectively, with respect to a C2 axis perpendicular to the principle axis -g and u are symmetric and antisymmetric with respect to an inversion centre (g is symmetric) |
|
|
Rationalise the spectrochemical series?
|
more electronegative => less σ- interaction => eg∗ is lower ∴t2g -> eg∗ gap is less π-donor< weak π-donor |
|
|
What is CFSE?
|
Crystal field stabalization energy is the energy gained by virtue of the relative stabalisation of the d electrons as a consequence of ligand binding. |
|
|
What is OSPE
|
the difference in crystal field stabalization energy for octaheral and tetratehral case (oct - tet) |
|
|
Spin selection rule? When is spin forbidden partially allowed? |
partially allowed by spin-orbit coupling |
|
|
Laporte selection rule? When is laporte partially allowed? |
partially allowed if no centre of symmetry (tetrahedron, vibrations in an octahedron) or if there is orbital mixing |
|
|
Charge transfer?
|
[ML₆]²⁺ + [ML₆]³⁺ -> [ML₆]³⁺ + [ML₆]²⁺ |
|
|
spin only formula? What does it represent? |
µ = √4S(S+1) since S = 1/2n where n is the number of unpaired electrons: µ = √n(n+2) predicted value of magnetic moment |
|
|
How do we get the spin only formula?
|
µ = √4S(S+1) + L(L+1) -orbital angular momentum is usually quenched µ = √4S(S+1) |
|
|
When is orbital contribution to magnetic moment not quenched?
|
when there is a partially filled t₂ or t2g orbitals. as they are related by rotational symmetry and a non-zero orbital contribution is expected when these rotations move an unpaired electron from one to another |
|
|
explain spin-cross over behaviour?
|
When spin pairing energy is approximately equal to ∆, at low temperatures tehy adopt low spin but at high temperatures adopt a metastable high spin (or when irradiated by a laser) due to a large activation barrier to returning to the ground state |
|
|
step wise stability constant
|
M + L -> ML => K₁ = [ML] / [M][L] ML + L -> ML₂ => K₂ = [ML₂]/[ML][L] etc (metals are rarely free ion so it is likely that the ligand is displacing solvent molecules that are surrounding the metal but that is usually ommitted) |
|
|
overall stability constant?
|
β₄ = K₁.K₂.K₃.K₄ |
|
|
Pattern for successive stability constats(+reasoning)?
|
-increasing number of the previously displaced ligand being in the solution (statistical factor) -sterics and electronic factors |
|
|
How is stability constant related to the free energy of the reaction
|
∆G⁰ = -RTlnβ
|
|
|
Factors effecting stability?
|
Charge - increasing charge on metal increases logK Steric effects- bulkier groups decrease logk |
|
|
Discuss M(PR₃)₄?
|
The four bulky phosphines are unable to fit around the metal centre and smaller ligands are favoured irrespective of π acceptor capability sometimes a PR₃ can be lost such as RHCl(PPh₃)₃ to form an unsaturated 14 electron intermediate
|
|
|
Define hardness?
|
I = ionisation potential, A = electron affinity |
|
|
What is symbiosis?
|
Modifing the properties of a metal ion using ancillary ligands
|
|
|
What are ancillary ligands?
|
Ligands that don't take part in the chemistry of a complex but help stabalize the complex or contribute steric or electronic effects such that the activity of a complex can be altered by altering the ligands |
|
|
Irving Williams order? |
Ca<<V<Cr>Mn<Fe<Co<Ni<Cu>Zn All 2+ |
|
|
What other trends follow the Irving Williams order?
|
hydration enthalpies, sum of the first and second ionisation potentials + other related reactions |
|
|
What is the Irving Williams series studying?
|
The equilibrium constants for the reaction of divalent metal ions with ethylene diamine
|
|
|
Rationalise the Irving Williams series?
|
-effects arising from CFSE (note dips at d⁵ and d¹⁰ corresponding to zero CFSE) -Stabilisation from Jahn-Teller stabilisation of Cu(II) which inceases the binding of the first four ligands/atoms at the expense of teh remaining two |
|
|
Stabilisation of High Oxidation states? +rationalise
|
F⁻, O²⁻ stabalize high oxidation states because of their small size(little steric effects), high electronegativity (large AB bond energy), good π-donor ability (resulting in a decrease in ∆₀) and very difficult to oxidise. |
|
|
Stabilisation of Low Oxidation states?
|
Stabalized by π-acceptor ligands such as CO, phosphines, because they donate σ-electrons into unoccupied metal p- or s-orbitals but accept electron density from filled metal d orbitals into unoccupied antibonding ligand orbitals (back-bonding)
|
|
|
What effect does backbonding have?
|
It strengthens the M-L bond but weakens bonds within the ligand itself, there is no build up of change on each atom as electron density donated by the ligand lone pair is returned by back bonding
|
|
|
18 valence electron rule?
|
Applies particularly to low oxidation state and covalent complexes |
|
|
Class 1 coordination compounds? Reasoning?
|
The ∆₀ is relatively small for 3d metals and for ligands at the lower end of the spectrochemical series t2g is non bonding (0-6 electrons) eg is weakly antibonding (0-4 electrons) ∴12-22 valance electrons due to small value of ∆tet tetrahedral complexses fall into this category also |
|
|
Class II coordination compounds? reasoning?
|
18 electron rule is never exceeded -∆o is larger for 4d and 5d metals and for σ ligands relatively high in the spectrochemical series -t2g is still essentially non-bonding (0-6 electrons) -eg is antibonding and is no longer available for occupancy -similar for 3d metals with ligands with very high ligand field strength (like CN-) |
|
|
Class III coordination compounds? reasoning? |
-∆o is largest for ligands at the uppermost end of the spectrochemical series -t2g becomes bonding due to interactions with π∗ orbitas and occupied by 6 electrons -eg is strongly antibonding and is empty -18 is obeyed unless steric factors prevent it -most low oxidation state organometallic compounds are of class III |
|
|
What sort of metal iosns form M-M bonds?
|
low oxidation state metals ions in which there is plenty of electron density to form overlap between the metal centres |
|
|
Trends in M-M bonding?
|
tendency towards metal-metal bonding increases descending a group as higher members tend to repel one another |
|
|
Mo diagram for determining M-M bonding?
|

|
|
|
How is free energy related to the standard electrode potential?
|
where F is faradays constant |
|
|
Discuss electrode potentials with magnitudes >1?
|
The oxidised form of a compound with a large positive electrodes potentials is a powerful oxidising agent |
|
|
Disuss low or negative values of electrode potential?
|
The reduced form of a compound with a low or negative electrode potential is a reducing agent
|
|
|
Trends in number of oxidation states?
|
-increases for the first 6 transitoin metals for second and third row then decreasing after |
|
|
Trends in relative stability of higher oxidation states for 3d? |
decrease from Sc to Zn |
|
|
Trends in electropositivity?
|
middle - little difference down the group right - become more noble (increasing electrode potential) |
|
|
What is plotted on a frost diagram?
|
∆G⁰/F (=-nE⁰)is plotted on the y axis and oxidation number is plotted on the x axis |
|
|
What conclusions can be obtained from a frost diagram?
|
A species on a convex curve will tend to disproportionate A species on a concave curve will not disproportionate A species which is high and on the right will tend to be strongly oxidising A species which is high and on the left will tend to be strongly reducing |
|
|
Disproportionation vs comproportionation?
|
Comproportionation is the opposite - V²⁺ + VO²⁺ -> V³⁺ |
|
|
Discuss Chromium triad?
|
-Mo most stable is +4 and W is +6, Cr is +3 |
|
|
What is a Pourbaix diagram?
|
a Potential vs pH diagram which maps out possible stable (equilibrium) phases of an aqueous electrochemical system |
|
|
Define Kinetics terms for rate of reaction?
|
labile - reacts quickly |
|
|
Thermodynamic terms relating to whether something will react or not?
|
Unstable -> easily reacts |
|
|
What factors affect lability?
|
Chelate effects size charge d-electrons what row of the transition metal you are in Jahn Teller effects |
|
|
How does chelate effects affect lability?
|
chelating ligands are thermodynamically more stable due to entropic effects (less required for each metal centre) so the activation energy for a ligand replacement is higher so lability is decreased |
|
|
How does charge affect lability?
|
metals with higher charges are stronger lewis acids, and so bind ligands more tightly making it less labile |
|
|
How does size of the ion effect lability?
|
larger ion have higher coordination number making them more labile |
|
|
How does moving down the d block effect lability?
|
Second and third row transition metals are much more inert than first-row transition metals as they have much larger d orbital splitting energies than the first-row metals due to more diffuse orbitals forming stronger bonds to ligands |
|
|
How does d orbital splitting diagram affect lability? |
-electrons in t2g orbitals are mostly non-bonding but not having empty orbitals complicates the donation of the substituting ligands electrons decreasing lability -no d electrons at all increases lability as there are empty d-orbitals for ligands to donate electrons into |
|
|
How does high spin vs low spin affect lability?
|
high spin electrons are in the eg orbital which is anti bonding making bonds to ligands weaker increasing lability |
|
|
Jahn Teller effects affect on lability?
|
makes Cu more labile because of the weaker ligands on the z axis
|
|
|
Crystal field activation energy?
|
CFAE = CFSE(oct) - CFSE(SquarePyramid)
|
|
|
What does FCAE tell us about lability?
|
The higher the FCAE the more inert the compound is low spin are more inert than high spin |
|
|
Mechanisms of Ligand Exchange Reactions?
|
A, D, Ia, Id |
|
|
Discuss Associatively activated reactions?
|
LnMX + Y -> LnMXY -> LnMY + X Rate constant has small dependence on leaving group and a large dependence on entering group |
|
|
Discuss dissociatively activated reactions?
|
LnMX + Y -> LnM + X + Y -> LnMY + X Rate constant has a large dependence on leaving group and no dependence on entering group |
|
|
Discuss the interchange mechanism?
|
LnMX + Y -> X----LnM----Y -> LnMY + X Two types |
|
|
Intimate mechanism?
|
How the bonds are formed and broken and information about the shape of the energy profile
|
|
|
Stoichiometric mechanism?
|
The sequence of elementary steps i.e. R + R' -> R-R' -> ...-> ... |
|
|
Associative interchange?
|
Ia: intimate mechanism where entering group is bound strongly and rate is sensitive to entering group but not very sensitive to leaving group. implying that the entering group and leaving group are strongly bound in the transition state |
|
|
Dissociative interchange?
|
Id: The rate is not sensitive to entering group but is sensitive to leaving group. Implying that the groups are weakly bound to the transition state |
|
|
What type of mechanisms do different types of metals tend towards?
|
-larger metals (4d, 5d) lean towards Ia -low d electron density encourages partly Ia characteristics |
|
|
How can be get information about the mechanism of an inorganic reaction?
|
-rate law gives us teh composition of the transition state species i.e. teh stoichiometric mechanism -variation of rate constant with structure and substituent gives the intimate mechanism |
|
|
Effect of peripheral ligands on the rates of substitution reactions?
|
Trans effect: where a ligand make a second ligand trans from itself a better leaving group |
|
|
Explain the trans effect?
|
π-Component - if T is better at π-bonding than the rest of the ligands then it is more likely to take up an equatorial position in the trigonal bipyramidal 5 co-ordinate intermediate |
|
|
Classes of electron transfer mechanisms?
|
outer sphere mechanism |
|
|
Define inner sphere mechanism?
|
Mechanism in which the reductant and oxidant share a ligand in their inner (or primary) co-ordination sphere with the electron being transfurred across the bridging group |
|
|
Define outer sphere mechanism?
|
Mechanisms involving electron transfer from reductant to oxidant withthe co-ordination spheres of both remaining intact
|
|
|
Effectiveness of bridging ligands in inner sphere redox reactions?
|
ability to conduct electrons increases with ligand polarisability |
|
|
Factors affecting the rate of outer sphere redox reactions?
|
|
|
|
Marcus Cross relation?
|
kab = √kaakbbKabF Where Kab is the equilibrium constant and F is a correction term (often 1) for the difference in free energies of the two reactants |
|
|
equation with ∆G
|
= -RTlnK = ∆H - T∆S |
|
|
Two electron transfers?
|
transfer of two inner sphere electrons -complementary reactions - oxidised and reduced forms of both ions differ by two units -non-complementary reactions - one pair of species has an oxidation state differenc of 2 but the second has a difference of one requiring a multistep mechanism with a unstable intermediate |

