![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back

Give two reasons why the first standard ionisation energy for potassium is much less than that of argon (2 marks) Q4)i) Jan 2009 |
(1) (the electron is being removed) from an energy level further from the nucleus
(2) there is increased shielding for potassium
|
|

Give a reason why the value for the second standard molar ionisation energy of potassium is larger than that of argon (1mark) Q4)ii) Jan 2009 |
The nuclear charge is greater for potassium |
|
|
State how you would explain to the general public how the pH scale is used to describe levels of acidity (2 marks) Q5 Jan 2009 |
(1) Acidic solutions have a pH of less than 7. (2) The lower the figure the "higher" the degree of acidity |
|

Use the equation to explain why sulfur dioxide is described as an acidic oxide. (1mark) Q5)b)i) Jan 2009 |
(When sulfur dioxide reacts with water) hydrogen ions/ H+(aq) are produced. |
|
|
Explain what is meant by dynamic equilibrium. (1mark) |
The rate of the forward and backward reactions are equal. |
|

Use Lewis Chatelier principle to explain how the concentration of hydrogen ions, H+ (aq), would change if more sulfur dioxide were dissolved in a solution that had reached dynamic equilibrium (2marks) |
(1) the concentration of hydrogen ions/ [H+] would increase
(2) as an increase in the concentration of the reactants moves the position of the equilibrium to the right |
|
|
Give an example of a process that uses heterogeneous catalyst, stating the process and the name of the catalyst (2marks) |
(1) Process: Haber process (2) Catalyst: Iron / (1) Process: Contact process (2) Catalyst: Vanadium (v) oxide |
|
|
Define the term standard conditions (1mark) |
298K (25°C) and 1 atmospheric pressure/atm |
|
|
State Hess's Law. (1mark) |
The enthalpy change in a reaction is independent of the pathway taken. |
|

Explain why the spectrum is seen as a series of sharp lines and not as a continous spectrum. (2marks) (1QWC) |
(1) The energy levels are quantised/ only certain energy levels are possible
(2) therefore only certain frequencies are allowed
(3) QWC |
|
|
Explain the meaning of the term relative isotopic mass (2mark) |
(1) relative isotopic mass is a term that describes the number of times one isotope of an atom is as heavy.. (2) as 1/12 of a Carbon-12 atom |
|
|
Explain how the relative atomic mass differs from the relative isotopic mass. (1mark) |
Relative isotopic mass only considers one isotope, but the relative atomic mass considers a weighed average of all the isotopes present. |
|
|
State how you would identify the endpoint of a titration. (1mark) |
(1) By using an indicator Eg. Methyl orange/ Methyl red/ phenolpthalein ...or (Can accept) (2) use a pH meter |
|
|
Explain the meaning of the term atomic number (1mark) |
Atomic number is the number of PROTONS in the nucleus. |
|
|
Explain the meaning of the term Isotope (1mark) |
The isotope of elements have the same number of protons but different number of neutrons/ mass number |
|
|
Explain how a catalyst speeds up a reaction (2marks) |
(1) it provides a new route (2) of lower activation energy |
|
|
State the two types of catalysts. (2marks) |
(1) Homogenous (2) Heterogenous |
|
|
Write an equation for the acid-base reaction of ammonia with sulfuric acid (1mark) |
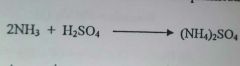
|
|
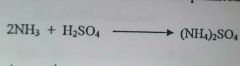
Explain why ammonia (NH3) behaves as a base in this reaction (1mark) |
Ammonia accepts a proton from the (sulfuric) acid/ Ammonia has a lone pair of electrons/ Ammonia neutralises the acid (Any one for the 1mark) |
|

Explain why hydrogen emits only certain definite frequencies of visible light. (2mark) |
(1) only changes between energy levels allowed/ Electron falls from higher energy levels to lower energy levels. (2) Energy emitted related to frequency/ E=hf/ the difference between any two energy levels are fixed/ energy levels are quantised. |
|
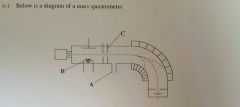
Name parts B and C of the mass spectrometer (2mark) |
(1) B = Electron gun/ source of electrons/ heated filament.
(2) C = Electric field/ charged plates/ accelerator/ collimator |
|
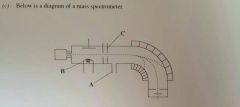
State the function of part A (1mark) |
To ensure a vacuum/ prevents collisions between sample and air molecules |
|

|
|
|

Give 2 reasons why the use of gas to generate electricity doesn't match the definition of sustainability. (2 marks) (1QWC) |
(1) Gas increase CO2 levels/ causes global warming.
(2) Gas is a non-renewable source of energy/ will run out.
|
|
|
Suggest one method of generating electricity which would be sustainable and outline how it works (2marks) |
(1) Method : (2) How it works
Wind : Rotation of blades turns turbine. Hydro : Falling water turns turbine. Geothermal : Combustion steam turns turbine. Solar : Sunlight on photovoltaic cell produces electricity. (Accept answers in terms of energy changes) |
|
|
In some countries, ethanol is replacing petrol (octane) as a car fuel.
Suggest a reason why ethanol is being used rather than petrol. (1mark) |
Ethanol is a renewable fuel (if obtained by fermentation)/
Ethanol is cheaper in countries with plentiful sugar cane growth/
Ethanol is more carbon neutral/
Ethanol burns more cleanly. |
|
|
State the common sources of error in titration experiments (2marks) |
(1) funnel left in burette (2) air in pipette (3) not reading meniscus (4) solution in flask not mixed thoroughly (5) all of the solid not used to make solution |
|
|
Complete the following definition of the mole. (1mark) A mole is the amount of material containing the same number of particles as there are atoms in____________________________. |
... 12grams of the Carbon-12 isotope. |
|
|
Explain how you would tell, from the properties of the system, that equilibrium has been reached. (1mark) |
A chemical system is in equilibrium when: There is no change in the amount of each species present/ There is no change in the concentration present/ The physical (macroproperties) properties are constant. |
|
|
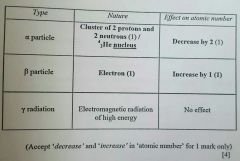
Explain what is meant by Beta- emission. (1mark) |
Loss of electron from the nucleus |
|
|
Explain the term half-life (1mark) |
Half-life is the time taken for half the amount of material to decay. |
|
|
State Le Chatelier's Principle. (1mark) |
When a system in equilibrium is subjected to change, the process which occur are such as to oppose the effect of the change. |
|
|
Define the term acid. (1mark) |
A proton donor |
|
|
Explain how CO2 behave as an acid when mixed in water. (1mark) |
(Although CO2 doesn't contain any hydrogen) it reacts with water to produce H+ ions/
To form carbonic acid/ H2CO3 |
|

|

(2) activation energy is the minimum energy required for a reaction to occur.
(3) increasing temperature increases the kinetic energy of molecules...
(4) ..so more molecules have greater energy than the activation energy (and the reaction speeds up).
(5) a catalyst lowers the activation energy...
(6) so speeds up the reaction. (7+8) QWC : SPaG + Presentation |
|
|
How would equilibrium yield be affected if the reaction were run without a catalyst? (1mark) |
Unchanged. |
|
|
A member of the public read in an article that the pH of an ammonium sulfate solution was 6. He asked you to explain what was meant by the pH scale.
What would be your reply? (2marks) |
(1) the pH scale is a measure of acidity/alkalinity.
(2) values below 7 are acidic and above are alkaline/ pH 7 is neutral/ pH 6 is a weak acid. |
|
|
Explain what is meant by the term 'heterogenous' (1mark) |
A reaction where the catalyst is in a different physical state to the reactants/products |
|
|
Explain what is meant by the term 'homogenous' (1mark) |
A reaction where the catalyst is in a SAME physical state to the reactants/products |
|
|
State the equation linking energy and frequency (1mark) |
E=hf Energy = plancks constant × frequency |
|
|
The atomic emission spectrum of hydrogen consists of several series of lines.
(i) Explain how these lines are formed. (3marks) |
(1) Lines represent the energy emitted...
(2) .. when an excited electron drops back...
(3) from one energy level to another. |
|
|
The atomic emission spectrum of hydrogen consists of several series of lines.
(ii) State the significance of the frequency of the convergence limit in the Lyman series. .(1mark) |
This represents the energy needed to remove the electron from the hydrogen atom. |
|
|
The atomic emission spectrum of hydrogen consists of several series of lines. (iii) Explain why there is more than one series of lines (1marks) |
In each series the excited electron drops back to a different energy level |
|
|
Suggest two factors that manufacturers should take into account when considering the production of maleic anhydride. (2marks) |
Any 2 of the factors: (1) use renewable energy resources (2) keep energy use to a minimum/low temperature/low pressure (3) use the most effective catalyst (4) use nontoxic materials wherever possible (5) the co-products should be nontoxic. (6) use renewable feedstocks (7) re-use/ recycle waste product (8) ensure high atom economy
|
|
|
Explain the term 'relative atomic mass' (2marks) |
(1) Average mass of one atom of the element... (2) ..relative to 1/12th the mass of one atom of Carbon-12 |
|
|
Explain the term 'molar first ionisation energy' (2marks) |
(1) Energy required to remove 1 mole of electrons from 1 mole of atoms... (2) ... in gaseous state. |

