![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
|
Give 8 risk factors of DM2 |
-increasing age: >40 if white, >25 if African-caribbean,black-African or south Asian -race: African-caribbean, black-African or south Asian -positive family history -high blood pressure -obesity – particularly central - BMI >30 -previous MI or stroke -schizophrenia, bipolar or depression if on anti-psychotic medication -female with polycystic ovaries, gestational diabetes or a babyweighing over 10 pounds -pre-diabetes |
|
|
Describe concordance in DM2 |
- 35-60%concordance in monozygotic twins - ~50% in dizygotic twins |
|
|
Name a gene which has been identified in genome-wide association studies to be linked to DM2. Briefly describe how it is linked to DM2 |
- TranscriptionFactor 7-Like-2 (TFF7L2) on chromosome 10q - encodes atranscription factor in the WNT signalling pathway |
|
|
What are the 2 main factors in the pathogenesis of DM2 |
- decreasedresponse of peripheral tissues to insulin (insulin resistance) - decreased ability of beta cells to produce insulin when combined withinsulin resistance and hyperglycaemia. |
|
|
Describe the 3 main implicaitons of insulin resistance |
(1)decreased uptake of glucose in muscle (2) reduced glycolysis andfatty acid oxidation in the liver (3) and an inability toprevent the liver from performing gluconeogenesis (i.e. becomesself-propagating) |
|
|
What are NEFAs and how are they implicated in insuoin resistance (essay answer)? |
-NEFAs: (1) INCREASED DEPOSITION IN TARET TISSUES (2) OVERWHELM FATTYACID OXIDATIO PATHWAYS PRODUCING TOXIC INTERMEDIATES (E.G.DIACYGLYCEROL AND CERAMIDE) (3) LEADS TO FAILURE IN THE BLOCKING OFENZYME, PHOSPHOENOLPYRUVATE CARBOXYKINASE, WHCIH LEADS TO AN INCREASEIN GLUCONEOGENESIS (4) INHIBITION OF GLYCOLYTIC ENZYMES NonesterifiedFatty Acids (NEFAs) - excess circulatingNEFAs are higher in obese people and linked with insulin resistance - obese people tend tohave higher levels of intracellular triglycerides in muscle and livertissue. This is likely due to excess NEFAs circulating and beingdeposited in these organs. - NEFAs tend to thedeposited in these more central sites, as these regions are moreactive in lipid metabolism – lipolysis - the significant ofexcess NEFAs, is that they overwhelm fatty acid oxidation pathways.This leads to the accumulation of toxic intermediates, such asdiacylglycerol and ceramide. - these toxicintermediates can activate serine/threonine kinases, interruptingserine phosphorylation on the insulin receptor and Insulin ReceptorSubstrates (IRS). Crucially, serine phosphorylation is the signallingmechanism by which insulin exerts its effect on the liver – thatfunction in this case being the inhibition of gluconeogenesis (byblocking the activity of the enzyme, phosphoenolpyruvatecarboxykinase. - by not blockingphosphoenolpyruvate carboxykinase, gluconeogenesis becomes increased– contributing to hyperglycaemia - NEFAs also compete withglucose for substrate oxidation, resulting in the inhibition ofglycolytic enzymes. Because glycolytic enzymes are inhibited, thereis an further increase in blood glucose |
|
|
Describe how adipokines are implicated in insulin resistance (essay answer) |
- adipose tissue is notjust fat storage, but a metabolically active organ that releaseshormones in response to different states - the proteins which arereleased from adipose tissue are called adipokines - some of these proteinspromote an increase in blood glucose (pro-hyperglycaemic adipokines)while others reduce it (anti-hyperglycaemia adipokines) - examples ofpro-hyperglycaemic adipokines include resistin and retinol bindingprotein 4 (RBP4) - examples ofanti-hyperglycaemic adipokines include leptin and adiponectin. Bothleptin and adiponectin improve sensitivity to insulin by enhancingthe activity AMP-activated protein kinase (AMPK) – an enzymeresponsible for fatty acid oxidation and glucose regulation in theliver and skeletal muscle. - crucially, adiponeticis reduced in obese people – contributing to insulin resistance (- AMPK is the target formetformin) |
|
|
How is inflammation linked to insulin resistance (essay answer)? |
- adipose tissue releasespro-inflammatory cytokines - this induces insulinresistance through cellular stress, activating signal cascades whichimpair the action of insulin on other tissues - notable examplesinclude: TNF alpha, interleukin 6 and macrophage chemoattractantprotein 1 - reducing the levels ofcytokines has been shown to improve insulin sensitivity |
|
|
What is PPARy and how is it linked with insulin resistance (essay answer) |
Peroxisomeproliferator-activated receptor y (PPARy) - PPARy is nuclearreceptor and transcription factor found on adipose tissue - it plays a crucial rolein adipocyte differentiation - mutations in the PPARgene cause a loss in protein function, resulting in certain forms ofmonogenic diabetes - PPARy is the target forthiazolidinedions. Activation promotes the production ofanti-hyperglycaemic adipokines – like adiponectin – causing thedeposition of NEFAs to increase towards adipose tissue, not liver andskeletal muscle. |
|
|
Describe the role of beta cell dysfunction in the development of DM2 (essay answer) |
- initially, increasinginsulin resistance is countered by increasing insulin production bybeta cells - this compensationallows, for a period, normal blood glucose levels to maintained - eventually,compensatory mechanisms fail, leading to hyperglycaemia - some people withinsulin resistance maintain beta cell compensation for significantlylonger periods than others, suggesting than intrinsic genetic defectsin beta cell function may also part in the development of DM2 - evidence for this hasbeen found in allelic variants in the diabetogenic gene, TCF7L2,associated with reduced insulin production from beta cells –indicating a predisposition to beta cell failure - many of the mechanismsinducing insulin resistance are also responsible for beta celldysfunction. Notably, NEFAs,and the resultant impairment of insulinsignalling, are thought to result in toxicity to beta cells. - similarly, metformin,while enhancing AMP-activated protein kinase (AMPK), also enhancesbeta cell function – illustrating shared mechanisms in theirpathogenesis - the replacement of betacells with amyloid is also a common finding in people with longstanding DM2 – present in >90% of islets examined - some believe amyloidprotein toxic to islets in a similar way to amyloid plaques inAlzheimer's |
|
|
Give 10 clinical presentations associated with DM2 |
-polyuria -nocturia -polydipsia (thirst) -polyphagia (hunger) -weight loss -fatigue -blurred vision -parathesias (numbness) typically in lower extremities -yeast infections – particularly balanitis in men -slow healing sores -skin darkening – due to acanthosis nigricans from insulinresistance |
|
|
What macrovascular complications are common in people with DM2? |
- MI - stroke - peripheral vascular disease |
|
|
Describe the pathogenesis of macrovascular complications in DM2 (essay answer) |
-diabetes has a significant impact on the blood vessels due to thedeleterious effects of persistent hyperglycaemia and insulinresistance -this predisposes endothelial dysfunction, subsequently promotingatherosclerosis and other CVD-associated com-morbidities -apart form severity and age of onset, it is indistinguishable fromdiseases of large and medium-sized vessels seen in the absence ofdiabetes -because of this predispostion, MI is the most common cause of deathin diabetes, though other associated diseases of the vessels arecommon also: renal vascular insufficiency and stroke -the increased risks also extend accordingly to pre-diabetics -in general, diabetics are 2-4 times more likely to suffer coronaryartery disease, and have a four fold increase in dying fromcardiovascular complication. -many people with diabetes also have hypertension – both due to thepresence of common, associated risk factors, but also due to thenature of DM pathogenesis. The increase in blood pressure exacerbatesthe damage caused by hyperglycaemia and insulin resistance 75% ofdiabetic patients will have hypertension. -insulin resistance may also promote diabetic dyslipidaemia, causingan increase in LDL over HDL – further exacerbating the risk ofatherosclerosis. This is thought to be due to the production ofhepatic, “atherogenic” lipids being promoted, while uptake ofcirculating lipids is inhibited. -diabetics also have elevated levels of PAI-1 which is an inhibitor offibrinolysis. Because there is more of it, there is less lysis offibrin, meaning there is a incresed predisposition to plaqueformation. |
|
|
What % of DM2 patients have signs of nephropathy? Which racial groups are more susceptible to developing diabetic nephropathy? What is the first sign of diabetic nephropathy? |
- 30-40% - native Americans, Hispanics, and African Americans - microalbuminuria (30-300mg/day) |
|
|
Other than diabetic retinopathy, what other 2 eye condition are common in DM2? |
- glaucoma - cataracts |
|
|
What 2 trials were pivotal in illustrating the importance of maintaining glycaemic control? |
- DCCTtrial in 1993 and the UKPDS trial in 1998 |
|
|
Describe the pathogenesis of microvascular damage in DM2 |
-most tissues require insulin to take up glucose – retina, kidneysand nerves do not -this means glucose is able to transverse cell membrane, though itcan't get out -when glucose is present in excess, it enters something called thepolyol pathway -in this pathway, glucose is metabolised to sorbitol by aldosereductase -the accumulation of sorbitol is what causes the metabolic damage toblood vessels -also causes a reduction of NADPH for metabolism, leading to a buildup of ROS (oxidative stress) -increase in glycation also causes production of Advanced GlycationEnd-products -together, this causes cellular damage which precipitates the symptomsin the microvascular and retina |
|
|
Draw a diagram illustrating the pathogenesis of microvascular damage |
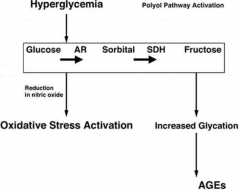
|
|
|
Describe the microscopic features and progrssion of diabetic retinopathy (essay answer) |
-hyperglycaemia causes damage tothe small vessel wall and microaneurysms - breaching of the vessel wall produces Dot haemorrhages
-protein and fluid are then left behind forming hard exudates -micro-infarcts then form – cotton wool spots -damage occurs to the veins which drain the retina, causing venousbudding and blockages -ischaemia leads to an increase in VEGF (and other growth factors),leading to neovascularisation, proliferative retinopathy (new, weakvessels) and vitreous haemorrhages -vessels burst causing build up of fluid in the macular area –macular oedema |
|
|
How is diabetic retinopathy prevented? |
-glycaemic control -stop smoking -control BP -address risk factors and have regular ophthalmic review (laser(removes new blood vessels), VEGF inhibitors and vitrectomy) -retinal screening is done annually from aged 12
-refer to ophthalmology is sight is threatened |
|
|
What are the 4 main stages of diabetic nephropathy in its progression? |
- renal enlargement and hyperfiltration - microalbuminuria - macroalbuminuria - end stage |
|
|
Describe the progression of diabetic nephropathy |
-damage cause small protein toleak through (microalbumiuria) -then progresses to macroalbuminuria -microalbuminuria involves only trace elements (30-300mg/24hours –normal: <20. Is a independent CV risk predictor. -as diabetes worsens, there is a increase in renal hypertrophy due toincreased GFR -the afferent arteriole then dilates (to reduce renal blood flow?)causing; increase in glomerular pressure, thickening of the GBM,capillary damage and shear stress on the endothelial cells -end result of this is leakage of protein - in later stages there is progressiveglomerulosclerosis, destruction ofglomeruli, increase proteinuria and then renal failure |
|
|
Describe diabetic nephropathy is prevented and treated? |
- screen formicroalbumiinuria (every year from diagnosis) - if present, treat withACE inhibitors and Angiotensin Receptor blockers to preventprogression to macroalbuminuria - after this, treatmentbecomes more aggressive in order to stop progression; - BP<125/75 - statins - smoking cessation - glycaemic control needsto be further improved (<7%) - refer to renal clinicif GFR falls <30 |
|
|
What are the 4 types of diabetic neuropathy? |
- peripheral (sensory)neuropathy – typically feet - autonomic neuropathy - mononeuritis multiplex - diabetic amyotrophy |
|
|
Describe the pathogenesis of diabetic neuropathy? |
- pathogenesis ofneuropathy essentially relates back to poor blood flow: blood flow tonerves is limited causing damage - impaired signalling ofsensory and motor nerves - increase in glucosecauses inability for nerve transmission of signals - causes metabolicchanges due sorbitol accumulation - causes vascular damagedue to capillary damage - results in structuraland functional changes |
|
|
Draw a diagram illustrating how hyperglycaemia precipitates peripheral neuropathy |
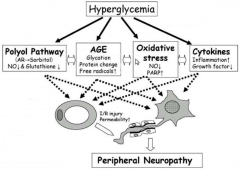
|
|
|
List 7 signs and symptoms of diabetic neuropathy |
- numbness - prickling - pain - reduced reflxes - preduced pressure sensation - reduced vibration sensation - burning pain |
|
|
How any amputations occur each week in the UK due to diabetes? Whta is the 5 and 2 year mortality of someone following amputation? |
- 100 - 80% and 50% |
|
|
Describe how the diabetic foot is classified? |
- low risk (normalsensation and pulse): annual review - medium risk (neuropathyor absent pulses): podiatrist every 3-6 months - high risk (deformity orulceration): podiatrist every 1-3 months |
|
|
What is Charcot foot and how is it caused? |
- essentially, numb feet - because of this theysuffer from repetitive micro traumas and stress fractures - because the blood flowis so poor, patients have an increase in bone turnover and fragilebones – flat feet |
|
|
Describe how autonomic neuropathy may affect the CV system, genitourinary system and GI system |
- neuropathy involvingnerves of the cardiovascular system commonly result in posturalhypotension - in the genitourinarysystem, erectile dysfunction - in the GI system,gustatroy sweating (sweating after eating) and gastroparesis (poorgut motility) |
|
|
What is the diagnostic criteria for DM2? |

|
|
|
What is the best way to prevent microvascular disease (1)? What is the best way to prevent macrobascular disease (6) |
- glycaemic control - multiple factors: glycaemic control; lipid levels, hypertension; smoking cessation; asprin therapy; diet and exercise |
|
|
What is the ideal pre-prandial glucose and HbA1c levels for DM2 patients? |
- 90-130mg/dL - 6-7% |
|
|
What is the first to fourth line treatments for DM2? |
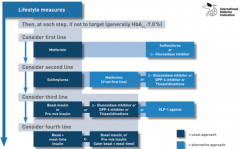
|
|
|
How do biguanides (metformin) work (3)? |
-lowers basal and post-prandial plasma glucose by reducing hepaticgluconeogenesis. - Also decreases intestinal absorption of glucose andimproves insulin sensitivity by increasing the peripheral uptake of glucose - generally well tolerated and doesn't promote weight gain orhypoglycaemia |
|
|
Give 2 examples of sulfonylureas How do they work? |
- glipizide and glimepride - insulin secretagogoues: stimulate the release of insulin from beta cells - may also enhance peripheral sensitivity by increasing the number of insulin receptors |
|
|
Name another class of insulin secretagogoues. How do they differ from sulfonylureas? Give 2 features which make them different to sulfonyureas |
- meglitinide derivatives e.g. repaglinide - shorter acting - lower risk of hyperglycaemia; higher risk of weight gain |
|
|
Give 2 examples of thiazolidinediones How do thiazolidinediones work (5)? |
- pioglitazone and rosiglitazone -function as insulin sensitisers – i.e. counteract insulinresistance -require insulin to be present to work -only antidiabetic agents proven to slow progression of diabetes –particularly in early disease -positive effect on reducing vessel inflammation though thoughassociated with oedema and weight gain -may have increased risk of MI |
|
|
How do selective Sodium-Glucose Transporter (SGLT-2) inhibitors work? What group of people might not be able to use this medication? |
-lower the renal glucose threshold – i.e. prevents the plasmaglucose concentration exceeding the glucose absorptive capacity ofthe kidney - those with renal failure |
|
|
Wat type of surgery might be offered for those with DM2? What group of people are most likely to be offered this? How much is this likely to affect blood ugar regulation? What are some of the potential negative outcomes (3)? |
- bariatric surgery - BMI >35 - returns to normal in 55-95% of patients - nutritional deficiencies, osteoporosis and death |
|
|
Give 2 examples of glucagon-like peptide 1 (GLP-1) agonists How do the work? What are some of their other advantages/disadvatages (3)? |
- exanatide and dulaglutide - mimic endogenous GLP-1: stimulate glucose-dependent insulin release,reduce glucagon and slow gastric emptying -associated with weight loss when combined with metformin andsulfonylurea; may prevent beta cell apoptosis and restore beta-cell mass (animalstudies); associated with thyroid C cell tumours and contraindicated in thosewith a family history |
|
|
How do dipeptidyl peptidase IV (DPP-4) inhibitors work? Give 2 examples |
-prolong the action of incretins: hormones that stimulate a decreasein blood glucose levels by causing an increase in the amount ofinsulin released from pancreatic beta cells after eating, beforeblood glucose levels become elevated - sitagliptin,saxagliptin |
|
|
Give examples of 2 insulins |
- lisproand aspart |
|
|
Describe how surgery can be used to treat DM2, including its advantages and disadvantages (4) |
-bariatric surgery is often performed in patient with DM2 with a BMI>35 -blood sugar levels return to normal in 55-95% of patients -drawbacks are its high cost and risks e.g. nutritional deficiencies,osteoporosis and death -also requires lifestyle adjustments |
|
|
Describe how diet and weight loss is used to treat DM2 |
-patients may continue eating the same foods though with calorificreduction -restriction of saturated fats and simple sugars is advisable -weight management, in general is the most important factor -the cost of foods should also be considered when advising patients aswell as cultural concerns -weight loss of 5-10% is associated significantly improved outcomesregarding CVD – via reduced HbA1c, blood pressure, increased HDL,and decreased plasma triglycerides. Weight loss of 10-15% isassociated with even greater outcomes -Mediterranean-style diets may be advantageous -Pasta enriched withbiologically active isoflavone aglycons improves endothelial functionin patients with type 2 diabetes mellitus and favorably affectscardiovascular disease risk markers |
|
|
List some of the checks and evaluations which need to be performed on diabetic people (6) |
- yearly dilated ey examinations - yearly microalbumin checks - foot examinations each visit - monitor for geriatric conditions (e.g. cognitive, vision, hearing, falls) - Parkinson's - pancreatic neoplasms |
|
|
What is the target blood pressure for those with DM2 (2)? What is the target blood pressure for those >1g/day proteinuria and renal insufficiency? What medication would you likely prescribe in a diabetic patient with high BP? |
- 140/80, though 130/80 is more suitable for younger patients - 125/75 - first line is ACE inhibitor though all would be viable dependng on the situation - e.g.angiotensin receptorblockers (ARB), diuretics, beta blockers, and calcium channelblockers |
|
|
Describe dyslipidaemia in DM2 patients and give 2 likely medications |
- high tryglcerides and low HDL - statins and fibrates |
|
|
Describe the treatment plan for a DM2 patient at risk of stroke (6) |
- screen BP regularly - >30 mins exercise a day - low sodium, high potassium diet, with low fat, fruits and vegetables - blood pressure target of <130/80mmHg - ACE inhibitors - statins |
|
|
What is the primary way of mananging ophthalmologic complications? |
- good glycaemic control |
|
|
Name 4 treatments for treating diabetic neuropathy |
- low dose tricyclic anti-depressants - duloxetine - anti-convulsants (e.g. phenytoin) - analgesics |
|
|
Name 6 infections/infectious conditions associated with DM2 |
-Malignant otitis externa -Rhinocerebral mucormycosis -Bacteriuria
-Pyuria -Cystitis -Upper urinary tract infection -Intrarenal bacterial infection -Skin and soft tissue infections -Osteomyelitis |

