![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
73 Cards in this Set
- Front
- Back
|
what are biological molecules?
|
the necessary molecules that make up all living things |
|
|
what common atom do organic molecules share and why?
|
the definition of an organic molecule is that it has carbon in it (with some exceptions) carbon is a very stable atom - due to its outer shell having only 4 electrons wants to complete its outer shell to satisfy the octet rule thus will to share electrons its nature is not to give away full or steal electrons - that would not be the optimal way to gain a total of 8 electrons in its out shell thus covalent sharing bonds are formed - unlike ionic bonds are not easy to break, carbon WANTS to keep those other 4 electrons shared since it forms stable bonds - makes sense that it would be used to form stable structures within an organism's body, needs structure also maximizes the number of bonds can be made (4 bonds versus just one, two etc.) |
|
|
be able to draw the atomic structure that prof put on the board - show sharing of electrons, bonds
|
one circle in the middle - number of + protons one ring around - 2 e- second ring around - 4 e- overlapping rings of another atom - draw a circle to designate each electron shared between the two overlapping rings |
|
|
what are the two main components of organic compounds?
|
string of carbons 2) functional groups attached to the carbon backbone provides molecule its characteristics |
|
|
what are common functional groups that are often attached to carbon backbones in organic molecules
|
found in alcohol, sugars 2)PO4 - phosphate group, positively charged, when it bonds creates high energy bonds 3) NH3 amine groups found in amino acids 4) COOH - carboxyl group (a carbonyl C=O and a hydroxyl group attached) |
|
|
what are monomers
|
|
|
|
how do monomers link together?
|
via dehydration synthesis - lose water molecule in the process |
|
|
how do monomers break apart
|
hydrolysis - add water to the bond
|
|
|
what are the four categories of biomolecules
|
2) lipids 3) proteins 4) nucleic acids |
|
|
what atoms are carbohydrates made of and what is the ratio of these atoms
|
1C, 2H, 1O |
|
|
what are the three most common atoms in the bio world
|
C, O and H |
|
|
what types of bonds exist in a carbohydrate?
|
C-H is a non polar covalent bond C-O is a polar covalent bond because O has higher electronegativity that C and H thus carbohydrates are POLAR MOLECULES, are HYDROPHILLIC in the bonds electrons are not shared evenly |
|
|
what are the monomers of carbohydrates called?
|
simple sugar |
|
|
how are sugars classified?
|
by how long their backbone are |
|
|
describe the structure of glycerol
|
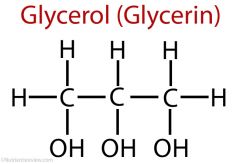
3 carbon sugar |
|
|
describe the structure of ribose/deoxyribose
|
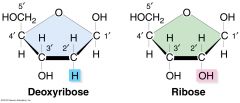
5 carbon sugar
|
|
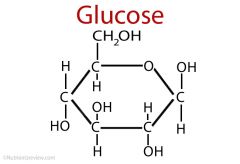
describe the structure of glucose
|
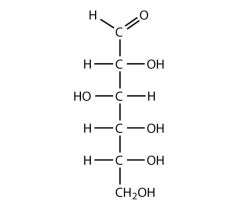
6 carbon sugar |
|
|
what functional group do carbohydrates mostly comprise of?
|
hydroxyl and carbonyls |
|
|
how does glucose get from its linear structure to its ring structure
|
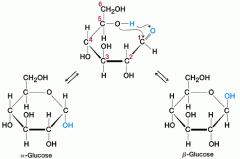
carbon 5's hydroxyl group loses a hydrogen, attacks the carbonyl group, binds to the carbonyl group's carbon creates a ring structure |
|
|
define the notation for drawing molecules
|
two lines meeting = a carbon atom a line with a blank space = hydrogen atom |
|
|
what are isomers
|
when other molecules have their atoms arranged in different ways but have the same molecular formula = isomers of each other |
|
|
what are isomers of glucose?
|
all have the molecular formula of C6H12O6 same functional groups taste sweet are also simple sugars |
|
|
what is a disaccharide?
|
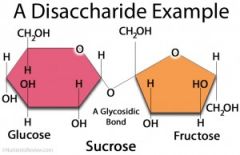
lose water, a hydroxyl and an H leave C is unstable without the OH, bonds to the O |
|
|
name the disaccharides
|
glucose + fructose = sucrose glucose + galactose = lactose |
|
|
what is a polymer of a carbohydrate called
|
long chains of monosaccharides bonded together via dehydration synthesis |
|
|
what is starch |
acts as an energy storage molecule for plants, animals can eat this and get energy from it as well |
|
|
what is cellulose
|
BRANCHED chains of glucose cannot be digested by animals also known as fiber, indigestible |
|
|
what is glycogen
|
stored in liver if you have high glucose intake and its not going to be used immediately, they link together within the animal multibranched polysaccharide of glucose |
|
|
what is chitin
|
monomer is N-Acetylglucosamine which is a derivative of glucose is NOT digestable |
|
|
what atoms are lipids made of
|
but do not occur in the same ratios as carbs have more C-H bonds |
|
|
explain why lipids are nonpolar (at least the tails are)
|
C-H share electrons evenly, thus nonpolar nonpolar molecules do not mix well interact with polar molecules, are hydrophobic |
|
|
name the 3 types of lipids
|
2) phospholipids 3) steroids |
|
|
describe the structure of a triglyceride
|
consists of a glycerol molecules and three fatty acid chains the polymer of a lipid large molecule is not complex |
|
|
what is glycerol
|
3 carbon molecule with 3 hydroxyl groups |
|
|
what is a fatty acid chain
|
called HYDROCARBON TAIL the carboxyl end of the fatty acid chain interacts with the hydroxyl group on the glycerol |
|
|
how do fatty acid chains bond to glycerol? |
via dehydration synthesis between the hydroxyl group and the carboxyl group |
|
|
what are the two different types of triglycerides?
|
all carbons have max number of hydrogens bonded saturated with hydrogens (2 per carbon) thus at room temp, they line up perfectly, solidly, bad for you 2) unsaturated not fully saturated with hydrogens because carbons double bonded to each other kinks thus at room temp, will not stack perfectly will instead be fluid at room temp, oils |
|
|
describe the structure of a phospholipid
|
are triglycerides but with phosphate groups attached to one of the hydroxyl groups of the glyecerol last OH does not add a FA but a phosphate group |
|
|
what is a phosphate group
|
negatively charged polyatomic ion |
|
|
describe the polarity of the phospholipid |
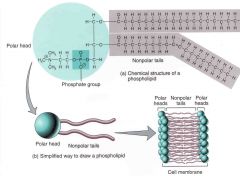
the fatty acid tails are hydrophobic thus the entire phospholipid molecule has a polar movement a dual nature molecule |
|
|
what happens when phospholipids are put into water |
cannot fully dissolve in water |
|
|
what is an everyday example of a common micel
|
thus fats other nonpolar molecules will go into the micel to cling to the phobic areas of the micel, while heads are able to cling to water, so will wash away and bring the nonpolar molecules with it they break up fat, lipids in water, act as emulsifiers |
|
|
what is a natural emulsifier? that uses the principle of the micel
|
produced in the liver, stored in the gallbladder bile is made of phospholipids, its hydrophobic tails surround the fat, breaking it apart into smaller globs of fat able to surround the fat with water, since their heads are hydrophillic |
|
|
what is the purpose of bile
|
when you eat very greasy foods they go into an aqueous environment within the stomach to break up the fats, for enzymes to get to them |
|
|
what are the structures that phospholipids can be found in
|
2) micel |
|
|
describe the structure of a phospholipid bilayer
|
the two sets of tails will face each other while the heads will face towards the water |
|
|
where does the phospholipid occur in nature |
the phospholipid bilayer will the curve around to form a sac the cell so that the cytoplasm can be aqueous, the outside of the cell is obviously aqueous and the middle of the bilayer is hydrophobic, nonpolar |
|
|
explain how the nonpolar middle layer of a phospholipid bilayer is important in maintaining the structure's integrity?
|
will naturally form together, the hydrophobic regions try to reach each other DIFFICULT TO FALL APART IN WATER unlike glucose or some other molecule - if something were made of glucose could fall apart in water, not adverse to water, no hydrophobic side to counteract wants to avoid the water |
|
|
what are steroids
|
act as chemical messengers in the body |
|
|
what is the most diverse, largest group of biomolecules?
|
are many many many different types of proteins because have all different functions they need to fulfill |
|
|
what are the atoms that proteins are made of
|
NITROGEN |
|
|
what are the monomers of proteins? |
amino acids |
|
|
what is the structure of amino acids |
thus when amine and carboxyl group bind together, creates a...sort of carbon backbone R group is the variable group = thus why diversity of amino acids |
|
|
how many different amino acids are there
|
there are 20 amino acids |
|
|
what types of R groups are there |
small as hydrogen or large ring groups hydrophilic or hydrophobic |
|
|
how are amino acids linked together
|
via dehydration synthesis between the carboxyl group and the amine group the OH of the carboxyl group leaves and the H on the amine leaves -> water leaves C=O and NH are directly linked together creating PEPTIDE BOND which is found only in proteins |
|
|
BUIRET'S REAGENT
|
amino acid won't have peptide bonds between them unless of course they somehow were made into proteins |
|
|
what is a peptide
|
small chain of amino acids linked together |
|
|
what is a protein in relation to a peptide
|
hundreds of amino acids polymers made of amino acids |
|
|
what are the 4 components of a protein's structure |
2) secondary structure 3) tertiary structure 4) quaternary structure |
|
|
what is primary structure |
simplest organization of a protein * order of this linkage, types of amino acids involved, are very important* because will affect later structure |
|
|
what is secondary structure |
amino acids interact with each other to from these preliminary 3D structures water interacts with the backbone to fold it into secondary structures |
|
|
what is a tertiary structure
|
the water outside is hydrophilic, some R groups are hydrophobic to the outside, this encourages folding |
|
|
what are the two types of secondary structures and how are they formed?
|
beta sheet - stair case structure formed via hydrogen bonding between the surrounding water molecules and the carbon-nitrogen backbone as well as hydrogen bonding between the carboxyl group and amine group on the backbone |
|
|
what are the 4 ways that R groups interact to produce protein tertiary structure
|
2)hydrogen bonding (polar R groups hydrogen bond to each other or to the outside water) 3) disulfide bonds (S atoms on the R groups covalently bonding to each other) 4)IONIC INTERACTION - some R groups have +/- charges |
|
|
what is quaternary structure
|
more than one peptide subunits joining together -> create an overall functional protein -> quaternary structure peptide subunits would have their secondary and tertiary structures formed |
|
|
proteins are defined as the workhorse of your body, why
|
due to all the R groups, interactions, layers of structures thus a huge variety of functions they can do enzymes, structure, accomplish everything in your body |
|
|
modern day prediction of protein structures?
|
can determine how they interact with each other in water or under certain conditions -> predict what their overall structures are find new uses for them, where the fit in biologically, what other proteins do they interact with |
|
|
nucleic acids are made of what atoms |
all atoms we use in biology |
|
|
what are the monomers of nucleic acids called |
bind together to make nucleic acids - polymers |
|
|
what is a nucleotide made of describe it structurally |
2) phosphate group 3) nitrogenenous base/nucleobase with the sugar at the middle, bonded to phosphate group on one side, nitrogenous base on the other side |
|
|
what does RNA stand for, DNA?
|
deoxyribonucleic acid |
|
|
|
|

