![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
MCAT expertise: the MCAT likes to present complex, novel molecules and then test you on the most basic information about them. Therefore, when dealing with carbohydrates on the exam,... |
look for the functional groups we have seen before (aldehydes, ketones, and alcohols) and realize that they retain the same chemical properties that you already know. |
|
|
Sugars with aldehydes as their most oxidized group are ______; sugars with ketones as their most oxidized group are _____. |
Sugars with aldehydes as their most oxidized group are aldoses; sugars with ketones as their most oxidized group are ketoses. |
|
|
Sugars with the highest-numbered chiral carbon with the -OH group on the right are D-sugars; those with the -OH on the left are L-sugars. D- and L- forms of the same sugar are enantiomers. |
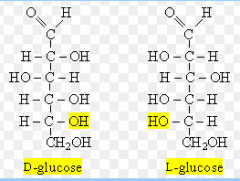
|
|
|
_____ are a subtype of diastereomers that differ at exactly one chiral carbon. _____ are a subtype of epimers that differ at the anomeric carbon. |
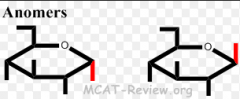
Epimers are a subtype of diastereomers that differ at exactly one chiral carbon. Anomers are a subtype of epimers that differ at the anomeric carbon. |
|
|
The most _____ carbon always gets the number 1 in numbering. |
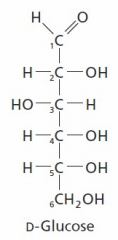
oxidozed |
|
|
Key concept: when trying to figure out how many possible stereoisomers can exist for a multiple carbon compound, identify the number of chiral carbons (n) and plug into the formula: |
2^n |
|
|
The _______ carbon is the new chiral center formed in ring closure; it was the carbon containing the carbonyl in the straight-chain form. β goes up, α goes down. |
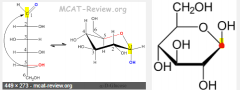
anomeric carbon |
|
|
Cyclic compounds can undergo _________, in which they shift from one anomeric form to another with the straight-chain form as an intermediate. |
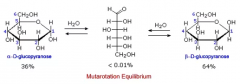
Cyclic compounds can undergo mutarotation, in which they shift from one anomeric form to another with the straight-chain form as an intermediate. |
|
|
Sugars that can be oxidized are reducing agents themselves (reducing sugars), and can be detected by reacting with Tollen's or Benedict's reagents. |
-A positive Tollen's test reduce Ag+ (silver cation) to metallic silver -A positive Benedicts test is indicated by a red precipitate |
|
|
-Sugars can react with carboxylic acids and their derivative, forming esters (_________). -________ is a similar reaction in which a phosphate ester is formed by transferring a phosphate group from ATP onto a sugar. |
-Sugars can react with carboxylic acids and their derivative, forming esters (esterification). -Phosphorylation is a similar reaction in which a phosphate ester is formed by transferring a phosphate group from ATP onto a sugar. |
|
|
______ formation is the basis for building complex carbohydrates and requires the anomeric carbon to link to another sugar. |
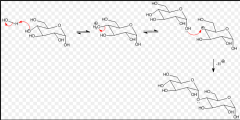
Glycoside formation |
|
|
Common disaccharides include _____ (glucose-fructose), _____ (galactose-glucose), and _______ (glucose-glucose) |
Common disaccharides include sucrose (glucose-fructose), lactose (galactose-glucose), and maltose (glucose-glucose) |
|
|
Polysaccharides: -______ is the main structural component for plant cell walls and is a main source of fiber -_____ (amylose and amylopectin) function as a main energy storage form for plants -_____ function as a main energy storage form for animals. |
-cellulose is the main structural component for plant cell walls and is a main source of fiber -starches (amylose and amylopectin) function as a main energy storage form for plants -glycogen function as a main energy storage form for animals. |
|
|
_____ can be oxidized to reduce electron carriers to facilitate processes like oxidative phosphorylation. |
Sugars can be oxidized to reduce electron carriers to facilitate processes like oxidative phosphorylation. |
|
|
Amylopectin is more soluble in water than amylose because of its branched structure and the increased interaction with surrounding solution. |
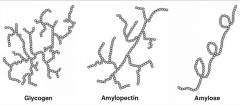
Glycogen has greater rate of enzymatic cleavage than amylopectin because of the higher frequency of branching. |
|
|
Difference between homopolysaccharide and heteropolysaccharide |
homopolysaccharide is a polysaccharide of the same monosaccharide. A heteropolysaccaride is a polysaccharide of different monosaccharides. |

