![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
76 Cards in this Set
- Front
- Back
|
Is oxygen electron withdrawing or donating?
|
withdrawing causing it to hold a slightly negative charge in a water molecule
|
|
|
what happens to a neutral molecule in water
|
water tend to weaken the interaction between the ions and thus promotes dissocation of the molecule into ions
|
|
|
T or F H bonds are covalent
|
FALSE
|
|
|
are H bonds considered strong or weak?
|
weak, but with a large amount of H bonds, pretty stable
|
|
|
_____ interactions are the dominant noncovalent forces encountered in most if not all biological systems
|
hydrophobic
|
|
|
If [H+] = 10e-4 what is the ph?
|
4
|
|
|
If [OH-] = 10e-4 what is the pH?
|
10, because [H+] would be 10e-10, so pH is 10
|
|
|
a change by one number in pH is actually a change by a factor of ____
|
10
|
|
|
[H+]x[OH-] =
|
10e-14
|
|
|
Keq =
|
[B-][H+]/[HB]
|
|
|
pH =
|
pK + log[HB]/[B-]
or pK + log[B-]/[HB] |
|
|
how do you define pK'
|
the pH at which an acid is 50% ionized
|
|
|
keq =
|
[B-][H3O+]/[HB]
|
|
|
what makes up a buffer
|
a weak acid AND it's conj base
|
|
|
what is the rule of thumb for when a buffer is a good buffer
|
when it is within one pH of it's pK value
|
|
|
what are the four major components of any amino acid
|
alpha amino group, R group, alpha carbon, alpha carbonyl
|
|
|
what is the three letter abbrev for asparagine
|
Asn
|
|
|
Three letter abbrev for glutamine
|
gln
|
|
|
Name the nonpolar aa
|
GAVIL P FWM
gly ala val ile leu pro phe trp met |
|
|
name the polar aa
|
STNQCY
ser thr Asn Gln cys tyr |
|
|
Name the acidic aa
|
asp glu
|
|
|
Name the basic aa
|
lys arg his
|
|
|
The solubility of an amino acid is strongly influenced by what two things
|
size and polarity of the R group
|
|
|
are human proteins L or D
|
L
|
|
|
which are the two strongly basic aa
|
arg and lys.. his is very sensitive to pH around neutral pH
|
|
|
pK of alpha carboxyl
|
3
|
|
|
pK of beta carboxyl like asp and glu
|
4
|
|
|
What is an example of an imidazole? what is the pK?
|
His
6 |
|
|
Which sufhydryl aa has a pK of 8?
|
Cys
|
|
|
what is the pka of an 1 primary alpha amino free
|
8 N terminal only
|
|
|
what is pK of lys and tyr
|
10
|
|
|
what is the pK of 2 alpha amino like proline
|
9
|
|
|
which aa is an example of a guanido? what is the pK?
|
arg
12 |
|
|
what is another name for peptide bond?
|
series of amide linkages
|
|
|
is a peptide bond a covalent bond?
|
yes
|
|
|
T or F
|
when alpha amino and alpha carboxyl groups are joined to make peptide bonds they become amides and no longer have appreciable acid base properties
|
|
|
what do you call a protein less than 5000 daltons or less than 50 aa?
|
peptides
|
|
|
what do you call a protein with two amino acids
|
dipeptide
|
|
|
are peptides named C to N or N to C
|
N to C
|
|
|
the ______ of the aa is critical for the structure and activity of a protein
|
sequence
|
|
|
describe primary structure
|
sequence of aa
|
|
|
describe secondary structure
|
local folding
|
|
|
describe tertiary structure
|
global folding
|
|
|
describe quaternary structure
|
oligomerization state
|
|
|
how can you tell between a secondary and tertiary structure
|
secondary structure is primarily a result of the chemical nature of the peptide bond whereas tertiary structure is more influenced by interactions between R groups
|
|
|
why do peptide bonds in a secondary structure tend to be all in the same plane
|
the CN single bond has considerable double bond character which puts restraint and leads to a tran formation which is also planar
|
|
|
in an alpha helix, the oxygen atom of one residue interacts with the hydrogen on the amide of the aa ___ residues away
|
4-- this allows it to complete an almost full turn
|
|
|
A H bond is weak, so how is an alpha helix so stable?
|
many h bonds make it stable
|
|
|
each aa can participate in how many h bonds
|
2
|
|
|
segments of proteins that cross membranes often form long sections of ______ (alpha or beta) composed of hydrophobic aa residues
|
alpha helix
|
|
|
T or F. polypeptide chains in a beta sheet can be parallel or antiparallel
|
true
|
|
|
definition. particular sequences of aa that are evolutionarily conserved among proteins
|
motifs
|
|
|
supersecondary structures (motifs) are frequently orgnanized into compact regions of tertiary structure called _____
|
domains
|
|
|
what makes up tertiary structures? aka what characteristics
|
3D folding and domains
|
|
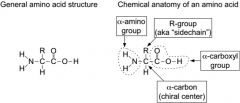
what is the general format of an aa?
|
see photo
|
|
|
what is the henderson hasselbach equation?
|
pH = pK - log(prot)/(unprot)
|
|
|
what does the HH equation tell you
|
calculate ratio of weak acid and conj base at any pH
|
|
|
aa are strung together by what kind of bond
|
peptide
|
|
|
If [HB]/[B-} = 100. what do you know?
|
that there is more HB in solution by 100 fold
|
|
|
can a peptide chain be formed by R groups between aa?
|
No, peptide bonds are formed using alpha carbon or alpha aa never the R group
|
|
|
why is a peptide bond planar?
|
due to resonance structure that restricts the number of conformations of a protein to si and phi
|
|
|
definiton. cluster of conserved residues
|
motif
|
|
|
what is the difference between conserved and variant
|
conserved is generally similar
invariant is always the same |
|
|
t or f. conserved parts of a protein tend to cluster
|
true. probably due to a very important function
|
|
|
definition. local foldings of residues into regular patterns
|
secondary structure
|
|
|
t or false a beta sheet cannot be twisted
|
false, beta sheets can be twisted -- beta turns!
|
|
|
are secondary structures limited to interprotein reactions?
|
no may be intra protein reactions too
|
|
|
what secondary structures do pro and gly tend to have
|
NO alpha helix and lots of beta turns
|
|
|
why doesn't glycine like to be an alpha helix?
|
thermodynamically disfavored. gly is small however and easily makes tight beta turns
|
|
|
why doesn't pro like to be alpha helix?
|
sterically disfavored. Small, kinked, easily makes tight turns.
|
|
|
definiton. global folding of protein chain
|
tertiary structure
|
|
|
definiton. higher order assembly of proteins
|
quaternary structure
|
|
|
what are three ways we can classify proteins?
|
functions definition
structural definition cellular localization definition |
|
|
what are four types of function definition proteins
|
enzymes
structural transport defense |
|
|
what are two types of structural definition proteins
|
globular
fibrous |
|
|
what are two examples of the cellular localization definition
|
membrane and soluble
|

