![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
Tosylate |
Alcohol with a large thing replacing the H |
|
|
Sn2 |
Substitution nucleophilic biomolecular: 2 molecules are involved in the rate determining step. |
|
|
Sn1 |
Substitution nucleophilic unimolecular: 1 molecule involved in the rate determining step. |
|
|
2nd order reaction |
Rate of reaction depends on concentration of both nucleophile and substrate. |
|
|
Benzylic |
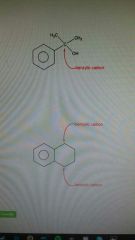
Carbon connected to a benzene ring. Good rate of reaction |
|
|
Allylic |
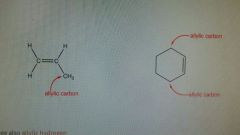
Carbon connected to a double bonded carbon. Have 3 carbons and 5 hydrogens Good rate of reaction. |
|
|
Vinyl |
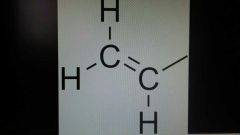
A group connected to a double bonded carbon. Have 2 carbons and 3 hydrogens. Absolute 0 rate of reaction. This is due to high electron density from pi bonds. |
|
|
Aryl |
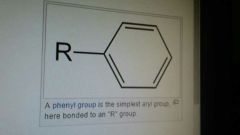
A group connected to a benzene ring. Absolute 0 rate of reaction. This is due to high electron density from pi bonds. |
|
|
Trends in nuclephilicity (Sn2) |
- Nuclephilicity decreases left to right ( Less EN, better nucleophile ) - Stronger bases, better nucleophiles - Nuclephilicity increases going up column - The presence of bulky groups on nucleophile will slow down rate |
|
|
Aprotic |
Elements that don't do hydrogen bonding. Smaller size, more nuclephilic. |
|
|
Protic |
Elements that do hydrogen bonding. The more size, the more nuclephilic. |
|
|
Why water is a good solvent? |
- Has both positive and negative ends. Can surround both positively and negatively charged ions. |
|
|
Factors for good leaving groups |
- More resonance = more stable/weak base - Larger size -> more reactive - EN -> better leaving group from left to right |
|
|
How to change leaving groups |
Adding acid to deprotonate the strong base to a better leaving group. |
|
|
Cyclization |
Leaving group is part of the same molecule as the nucleophile |
|
|
Lactone |
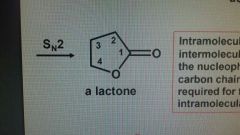
A cyclic ester |
|
|
1st order reaction |
Rate of reaction only depends on concentration of electrophile. Not nucleophile because it is not in the first step. |

